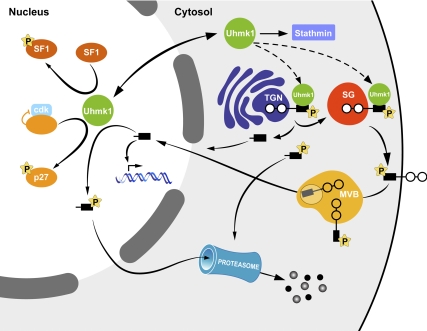
Figure 10.
Model outlining the LDCV/nuclear signaling pathway in which PAM and Uhmk1 participate. Under basal conditions, the Uhmk1 site in the CD of PAM (Ser949) is phosphorylated, expediting PAM entry into LDCVs; late stages in the endocytic trafficking of PAM require both phosphorylation and dephosphorylation at this site (8,10). The endoproteolytic cleavage that releases sf-CD is diminished in PAM with a phosphomimetic mutation at Ser949. In addition, Uhmk1 phosphorylation at this site reduces PAM-CD accumulation in the nucleus. Nuclear PAM-CD, acting through pathways that remain to be described, alters the expression of a limited subset of genes, including Aqp1, which is known to affect LDCV metabolism. In the nucleus, Uhmk1, which interacts with nuclear binding sites, can phosphorylate PAM-CD, leading to nuclear exit and rapid proteasomal degradation. PAM-1 with the phosphomimetic Asp949 mutation fails to traverse the endocytic compartment normally, fails to alter cytoskeletal organization or regulated secretion, and fails to generate sf-CD efficiently. Uhmk1 opposes many of the regulatory effects of PAM on LDCV metabolism; the factors that control Uhmk1 localization are unknown. P, Phosphorylation; SG, secretory granule.