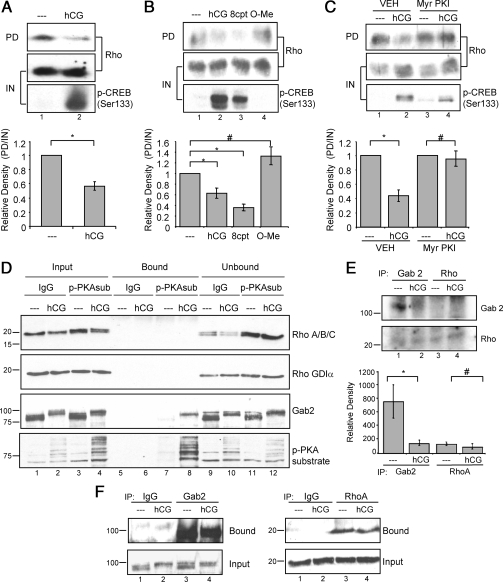
Figure 6.
LHR signaling via cAMP and PKA decreases Rho activity, but Rho is not phosphorylated directly by PKA. A, Preovulatory granulosa cells were left untreated (- - -) or treated with 1 IU/ml hCG for 10 or 30 min. Rho pull-down assays were performed as described in Materials and Methods. Western blots are representative of seven separate experiments. GTP-bound Rho levels were quantified by densitometric analysis of pull-down Western blot values divided by input to account for loading differences. Values for hCG-treated samples are expressed as a fold change from that of untreated controls, which has been set to a value of one. Values are the mean ± se of seven separate experiments. *, P < 0.001. B, Preovulatory granulosa cells were left untreated (- - -) or treated with 1 IU/ml hCG, 500 μm 8-cpt-cAMP (8cpt), or 500 μm 8-cpt-2′O-Me-cAMP (O-Me) for 10 or 30 min. Rho pull-down assays were performed as described in Materials and Methods. Western blots are representative of three separate experiments. GTP-bound Rho levels were quantified as described in A. Values are for hCG-treated or cAMP analog samples expressed as fold change from that of untreated controls, which has been set to a value of one. Values are the mean ± se of three separate experiments. *, P < 0.05; #, P > 0.05. C, Preovulatory granulosa cells were treated with ddH2O (VEH) or 50 μm Myr-PKI for 1 h and then left untreated or treated with 1 IU/ml hCG for 10 or 30 min. Rho pull-down assays were performed as described in Materials and Methods. Western blots are representative of three separate experiments. GTP-bound Rho levels were quantified as described in A. Values are expressed as a fold change from untreated controls with no inhibitor pretreatment. *, P < 0.01; #, P > 0.01. D, After treatment of preovulatory granulosa cells without or with 1 IU/ml hCG for 30 min, soluble cell extracts were subjected to coimmunoprecipitation using control IgG or anti-phospho-PKA substrate (p-PKAsub) antibody, as described in Materials and Methods. Input, unbound, and bound (immunoprecipitated) proteins were probed by Western blotting with indicated antibodies. The lower portion of the blot (below 37 kDa) was probed with RhoA/B/C and RhoGDIα antibodies; the upper portion of the blot was probed with phospho-PKA substrate and Gab2 antibodies. Molecular mass markers (kilodaltons) are indicated at the left. Input and unbound reflects 4% of total lysate; bound reflects 100% of immunoprecipitated proteins. Results are representative of four experiments. E, Granulosa cells were untreated or treated with 1 IU/ml hCG for 10 min. Soluble extracts were subjected to immunoprecipitation using antibodies to Gab2 or RhoA, and washed immunocomplexes were subjected to a back-phosphorylation assay in the presence of [γ-32P]ATP and the PKA catalytic subunit, as described in Materials and Methods. Results are representative of three separate experiments. Quantitation of signals is shown in the lower panel. *, P < 0.05; #, P > 0.05. F, After treatments of granulosa cells as described in E, soluble extracts were subjected to immunoprecipitation using indicated antibodies. Bound and input protein fractions were probed with indicated antibodies. Results are representative of 3 separate experiments.