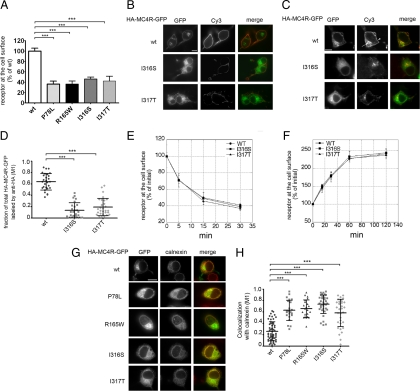
Figure 1.
Obesity-linked MC4R mutants are retained in the ER. A, N2A cells were transiently transfected with wt-HA-MC4R-GFP and mutated HA-MC4R-GFP (P78L, R165W, I316S, and I317T). HA-MC4R-GFP at the cell surface was measured by ELISA. Data are expressed as percentage of the wt receptor. B, Cells were transfected with wt-HA-MC4R-GFP and mutated HA-MC4R-GFP (I316S and I317T), transferred at 4 C, incubated with primary rat monoclonal anti-HA antibodies, fixed, and incubated with secondary Cy3-conjugated antirat antibodies. C, N2A cells were transfected as in B. Cells were incubated with rat anti-HA antibodies for 2 h at 37 C. Cells were fixed, washed with PBS, permeabilized, and incubated with Cy3-conjugated antirat antibodies. Arrow and arrowhead indicate endosomal compartment and plasma membrane, respectively. D, Quantification of the confocal images of the experiment shown in C. The wt-HA-MC4R-GFP (n = 30) and mutated HA-MC4R-GFP (I316S n = 31, I317T n = 30), M1, Mander’s overlap coefficient. E and F, N2A cells were transfected with wt-HA-MC4R-GFP and mutated HA-MC4R-GFP (I316S and I317S). Receptor internalization (E) and exocytosis (F) were measured as previously reported (18). G, N2A cells were transiently transfected with wt-HA-MC4R-GFP and mutant HA-MC4R-GFP (P78L, R165W, I316S, and I317T). Cells were permeabilized and stained with primary rabbit polyclonal anti-calnexin and secondary Cy3-conjugated antirabbit antibodies. H, Quantification of the colocalization of MC4R with calnexin was done using cells transfected with wt-HA-MC4R-GFP (n = 55) and mutated HA-MC4R-GFP (P78L n = 23, R165W n = 22, I316S n = 36, I317T n = 33). Bars, 25 μm. ***, P < 0.0001.