Abstract
Prolactin (PRL) is a multifunctional hormone with prominent roles in regulating growth and reproduction. The guinea pig (Cavia porcellus) has been extensively used in endocrine and reproduction research. Thus far, the PRL cDNA and protein have not been isolated from the guinea pig. In the present study, we used information derived from the public guinea pig genome database as a tool for identifying guinea pig PRL and PRL-related proteins. Guinea pig PRL exhibits prominent nucleotide and amino acid sequence differences when compared with PRLs of other eutherian mammals. In contrast, guinea pig GH is highly conserved. Expression of PRL and GH in the guinea pig is prominent in the anterior pituitary, similar to known expression patterns of PRL and GH for other species. Two additional guinea pig cDNAs were identified and termed PRL-related proteins (PRLRP1, PRLRP2). They exhibited a more distant relationship to PRL and their expression was restricted to the placenta. Recombinant guinea pig PRL protein was generated and shown to be biologically active in the PRL-responsive Nb2 lymphoma cell bioassay. In contrast, recombinant guinea pig PRLRP1 protein did not exhibit PRL-like bioactivity. In summary, we have developed a new set of research tools for investigating the biology of the PRL family in an important animal model, the guinea pig.
The guinea pig possesses an expanded prolactin family, including ligands produced by the anterior pituitary and placenta.
Prolactin (PRL) is a well-studied hormone/cytokine with a spectrum of biological roles across vertebrates (1,2,3,4,5). PRL proteins and cDNAs have been isolated from anterior pituitaries of a wide selection of species (6,7).
The guinea pig (Cavia porcellus) is an animal model that has been used extensively in reproduction and endocrinology research. Several guinea pig anterior pituitary hormone cDNAs and proteins have been identified and characterized, including those for glycoprotein hormones (8,9), proopiomelanocortin (10), and GH (11). Early reports suggested that the guinea pig anterior pituitary possesses PRL-like biological activity (12,13,14) and possibly PRL immunoreactivity (15). In some species, the PRL locus has been expanded and consists of a collection of genes encoding proteins structurally related to PRL (16). This expansion is most prominent in murine rodents and ruminants (16,17,18,19,20,21,22) and does not occur in other species such as the human and dog (23,24). To date, the PRL cDNA and protein have not been isolated from the guinea pig nor are there any insights about a possible expansion of the PRL locus in the guinea pig.
In the present study, we used information derived from the public guinea pig genome database as a tool for identifying guinea pig PRL. These efforts led to the characterization of guinea pig PRL and its expression and biological activity and the discovery and partial characterization of two guinea pig PRL-related proteins (PRLRP1, PRLRP2). Guinea pig PRL exhibits similarities in expression patterns and biological actions with other PRLs; however, it exhibits striking differences in structure when compared with PRLs of other mammalian species. The guinea pig possesses a unique expansion of the PRL family. Guinea pig PRLRPs are expressed in the placenta at differing levels. The more abundant PRLRP1 does not possess PRL-like bioactivity.
Materials and Methods
Animals and tissue preparation
Hartley guinea pigs were obtained from Charles River Laboratories (Wilmington, MA). The animals were housed in an environmentally controlled facility, with lights on from 0600 to 2000 h and allowed free access to food and water. Timed pregnancies were generated and tissue dissections were performed (25). The duration of gestation in the guinea pig is approximately 65 d. Tissues were frozen in dry ice-cooled heptane and stored at −80 C until used for histological analyses or were frozen in liquid nitrogen and stored at −80 C for subsequent RNA analyses. Protocols for the care and use of animals were approved by the University of Kansas Animal Care and Use Committee.
Cloning and characterization of guinea pig PRL family and GH cDNAs
Nucleotide sequences related to Prl were identified from guinea pig genomic sequences by BLAST (http://blast.ncbi.nlm.nih.gov) and BLAT (http://genome.ucse.edu/cgi-bin/hgBlat) analysis (version 2.2) (26) of the guinea pig genome assembly (cavPor2, http://www.ensembl.org/cavia_porcellus/index.html and http://genome.ucsc.edu/cgi-bin/hgGateway?org=Guinea+pig&db=cavPor3&hgsid=160847259). The guinea pig genome has been sequenced to 6.76X coverage with 95.55% of the bases assembled. Nucleotide sequences related to members of the PRL family were found on guinea pig. scaffold_38, _207, _13, _11, _108, _18, and _123. cDNAs for three members of the newly discovered guinea pig PRL family and guinea pig Gh were isolated by RT-PCR. Total RNA was extracted with the TRIzol reagent (27) from guinea pig pituitaries (Prl and Gh) and placentas (Prlrp1 and Prlrp2). Two micrograms of total RNA and 0.5 μg of oligo deoxythymidine (dT) were used for reverse transcription reactions. PCRs were performed using Platinum Taq DNA high-fidelity polymerase (Invitrogen, Carlsbad, CA) with sets of primers based on genomic sequences. PCR was performed for 30 cycles (denature, 95 C for 1 min; anneal, 55 C for 1 min; extension, 72 C for 1–1.5 min). Primer sequences used for amplification of Prl, Prlrp1, and Prlrp2 were based on guinea pig genomic sequences, whereas primer sequences used for amplifying Gh were based on a guinea pig Gh cDNA nucleotide sequence deposited in GenBank (accession no. AF233853). Primer sequence sets are presented in Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. The reactions were followed by a final elongation step of 10 min at 72 C. Amplified products were subcloned into pCRII-TOPO vector flanked by SP6 and T7 promoters with the TOPO TA cloning kit (Invitrogen). cDNAs were sequenced by the Northwestern Sequencing Facility of Northwestern University (Chicago, IL). The originally isolated PRL family cDNAs were not full length. Rapid amplification of cDNA ends (RACE) was used to identify 5′ and 3′ ends of the transcript (see below). Nucleotide and amino acid sequence comparisons were performed with CLUSTAL X (version 2.0; http://www.clustal.org/) (28). Multiple amino acid sequence alignments and phylogenetic tree construction were generated with CLUSTAL X (28). Locations of signal peptides were determined with the SignalP software program (version 2.0.b2; http://www. cbs.dtu.dk/services/SignalP/) (29) and based on homology with other members of the PRL family.
RACE analysis
Total RNA was isolated from guinea pig pituitary tissue and gestation d 35 placental tissue with TRIzol (27) for the characterization of Prl and the PRLRPs (Prlrp1 and Prlrp2), respectively. First-strand cDNA synthesis and subsequent amplification of 5′-cDNA and 3′-cDNA were performed as defined in the 5′/3′-RACE kit (Roche Molecular Biochemicals, Indianapolis, IN). The initial step in the 5′-RACE procedure involved first-strand cDNA synthesis using gene-specific primers for Prl (PRL-R2), Prlrp1 (PRLRP1-R2), or Prlrp2 (PRLRP2-R2). Primer sequences are provided in Supplemental Table 1. First-strand cDNAs were purified and homopolymeric A tails were added to the 3′ end of the cDNAs by terminal transferase. Tailed cDNAs were then subjected for the first PCR amplification using gene-specific primers for Prl (PRL-R3), Prlrp1 (PRLRP1-R3), or Prlrp2 (PRLRP2-R3) and the oligo dT-anchor primer. cDNA products were further amplified by a second PCR using nested gene-specific primers for Prl (PRL-R4), Prlrp1 (PRLRP1-R4), or Prlrp2 (PRLRP2-R4) and the PCR anchor primer. For 3′-RACE, first-strand cDNAs were synthesized by reverse transcription reaction using an oligo-dT primer. These products were then used to obtain the 3′ end of the cDNA by further amplification using a PCR-anchored primer and gene-specific primers for Prl (PRL-F2), Prlrp1 (PRLRP1-F2), or Prlrp2 (PRLRP2-F2). cDNA products for Prlrp1 and Prlrp2 were then generated by a second PCR using nested gene-specific primers (PRLRP1-F3, PRLRP2-F3). The resulting 5′-RACE and 3′-RACE products were subcloned and sequenced.
Analysis of mRNA
RT-PCR analysis of Prl family and Gh mRNAs
Prl, Prlrp1, Prlrp2, and Gh mRNAs were monitored by RT-PCR. Total RNA was isolated from an assortment of tissues. Five micrograms of total RNA and 0.5 μg of oligo dT were used for reverse transcription reactions. PCR was performed using Platinum Taq DNA high-fidelity polymerase (Invitrogen) with Prl-, Prlrp1-, Prlrp2-, or Gh-specific primers (Supplemental Table 1). PCR was performed for 30 cycles (denature, 95 C for 1 min; anneal, 55 C for 1 min for the PRL family members or 60 C for 1 min for Gh; extension, 72 C for 1 min). Amplified products (Prl, 488 bp; Gh, 800 bp; Prlrp1, 512 bp; Prlrp2, 418 bp) were resolved by electrophoresis in 1% agarose gels and ethidium bromide staining.
Northern blot analysis
Northern blots were performed as previously described (30). RNA was extracted using TRIzol (27) from a range of tissues, including anterior pituitary, ovary, mammary gland, testis, heart, lung, liver, kidney, spleen, placenta, and subplacenta. Total RNA (15 μg) was separated on 1% formaldehyde-agarose gels and transferred to nylon membranes. Blots were probed with [32P]-labeled cDNAs for guinea pig Prl, Prlrp1, or Prlrp2. An 18S cDNA was used as an internal reference to ensure integrity of the RNA samples.
In situ hybridization
Prlrp1 mRNA was detected in placental tissues using nonradioactive in situ hybridization as previously described (31). Ten-micrometer cryosections of tissues were prepared and stored at −80 C until used. A plasmid containing a cDNA for Prlrp1 was linearized and used as template to synthesize sense and antisense digoxigenin-labeled riboprobes according to the manufacturer’s instructions (Roche Molecular Biochemicals). Tissue sections were air dried and fixed in ice-cold 4% paraformaldehyde in PBS. Prehybridizations, hybridizations, and detection of alkaline phosphatase-conjugated antidigoxigenin were performed as previously reported (31). Images were captured using a Leica MZFLIII stereomicroscope equipped with a Leica charge-coupled device camera (Leica Microsystems GmbH, Welzlar, Germany).
Immunocytochemistry
Immunocytochemical analyses were used to localize PRL and GH proteins in the guinea pig pituitary. Cryosections (10 μm) were prepared, fixed in cold 4% paraformaldehyde solution, and blocked in 10% normal goat serum for 1 h at room temperature. Incubations were performed overnight at 4 C with antiporcine PRL antibodies (lot no. AFP7P; National Hormone and Peptide Program, Torrance, CA; 1:100 dilution) or antiporcine GH antibodies (lot no. AFP422801Rb; National Hormone and Peptide Program; 1:1000 dilution). Specificity of the immunoreaction with the antiporcine PRL antibodies was evaluated by coincubation with recombinant guinea pig PRL protein (50 μg/ml). Tetramethylrhodamine isothiocyanate-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO; 1:400 dilution) was added for 30 min at room temperature. Tissues were counterstained with 4′,6-diamidino-2-phenylindole (Molecular Probes, Carlsbad, CA). Images were captured using a Leica DMI 4000 microscope equipped with a Leica charge-coupled device camera.
Generation of recombinant guinea pig PRL and PRLRP1 proteins
PRL and PRLRP1 were expressed as fusion proteins with a FLAG-6xHis-FLAG tag. Full-length mature guinea pig Prl and Prlrp1 cDNAs were used as templates for PCR amplification of Prl and Prlrp1 fragments with EcoR1 and BamH1 restriction sites at the 5′- and 3′-ends, respectively. Amplified products were generated using sequence specific primers for Prl (Prl-F3 and Prl-R5) and Prlrp1 (Prlrp-F4 and Prlrp-R5) (see Supplemental Table 1). After digestion with EcoR1 and BamH1 restriction enzymes, the fragments were ligated into a modified pFLAG-CMV-3 vector (mFLAG; Sigma-Aldrich). The accuracy of vector construction was verified by DNA sequencing. The mFLAG-PRL and mFlag-PRLRP1 plasmids were transfected into human embryonic kidney cells (HEK-293) cells using Lipofectamine Plus according to the manufacturer’s instructions (Invitrogen). HEK-293 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM/F12 medium supplemented with 10% fetal bovine serum, 1 mm sodium pyruvate, penicillin (100 U/ml), and streptomycin (100 μg/ml). Initial selection of transfected cells was accomplished in the presence of G418 at a concentration of 500 μg/ml. Selected cells were then maintained in 100 μg/ml of G418, and serum-free conditioned medium was collected. The expression of mFLAG-PRL and mFlag-PRLRP1 fusion proteins was confirmed by Western blot analysis with anti-Flag M2 antibody (Sigma-Aldrich).
Western blot analysis
Lysates were prepared by homogenizing genetically engineered HEK-293 cells in radioimmunoprecipitation assay buffer (10 mm Tris-HCl, pH 7.2; 1% Triton X-100 or 1% Nonidet P-40; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 150 mm NaCl; 5 mm EDTA; 1 mm sodium orthovanadate; 1 mm phenylmethylsulfonyl fluoride; 10 μg/ml aprotinin). Protein concentrations were determined by the DC protein assay (Bio-Rad, Hercules, CA). Fifty micrograms of total protein were separated by SDS-PAGE and transferred onto nitrocellulose membranes. Guinea pig flag-tagged PRL and PRLRP1 were identified with anti-FLAG M2 antibodies (Sigma-Aldrich) and visualized by enhanced chemiluminescence according to the manufacturer’s instructions (Amersham Biosciences, Piscataway, NJ). Flag-tagged rat PRL-like protein-J (PLP-J) (32) and mock-transfected HEK-293 cells were used as positive and negative controls, respectively.
Nb2 lymphoma cell proliferation assay
The Nb2 lymphoma cell proliferation assay was performed with some modifications to the previously published procedure (33). The rat Nb2 lymphoma cell line was provided by Dr. Peter Gout (University of British Columbia, Vancouver, British Columbia, Canada) and maintained in RPMI 1640 culture medium supplemented with 10% horse serum (HS), 10% fetal bovine serum, 50 μm 2-mercaptoethanol, 2 mm l-glutamine, 5 mm HEPES, penicillin (50 U/ml), and streptomycin (50 μg/ml) (33). Twenty-four hours before initiating the assays, cells were incubated with RPMI 1640 supplemented with 5% HS, 2 mm l-glutamine, 5 mm HEPES, and antibiotics (assay medium) to establish a quiescent state. Cells were washed with assay medium, counted with a hemocytometer, and distributed to wells in a 96-well plate (2.5 × 104 cells/well). Cells were incubated with ovine PRL (NOBL Laboratories, Inc., Sioux City, IA), genetically engineered HEK-293 cell-conditioned medium, or control HEK-293 cell-conditioned medium from mock-transfected cells for 72 h. Viable cells were quantified by the CellTiter 96 AQuous nonradioactive cell proliferation assay (Promega, Madison, WI).
Statistical analysis
Statistical comparisons between two means were determined with Student’s t test. Comparisons among multiple means were evaluated with ANOVA. The source of variation from significant F ratios was determined with Bonferroni’s multiple comparison test (34).
Results
Identification and characterization of guinea pig Prl and Gh cDNAs
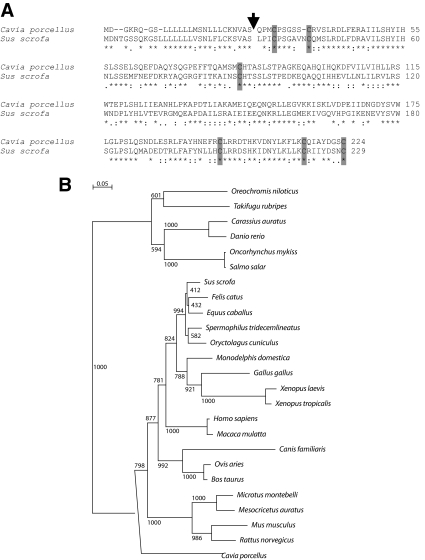
BLAST and BLAT analyses of the public guinea pig genome assembly (cavPor2, http://www.ensembl.org/cavia_porcellus/index.html and http://genome.ucsc.edu/cgi-bin/hgGateway?org=Guinea+pig&db=cavPor3&hgsid=160847259) with cDNA sequences for members of the PRL family resulted in the identification of a nucleotide sequence resembling Prl. Guinea pig Prl is a five-exon gene similar to the organization of other PRL family genes (7) and spans 6,904 nucleotides on the minus strand of guinea pig. scaffold_38 from nucleotide 16,887,325 to 16,894,229. PRL cDNAs were cloned by RT-PCR from guinea pig pituitaries, sequenced, and compared with vertebrate PRL sequences. Guinea pig Prl (GenBank accession no. GU929698) encodes a predicted protein of 224 amino acids, with a 26-amino acid signal peptide, and the mature protein possesses cysteine residues in locations homologous to the six cysteines found in other PRL proteins (Fig. 1). Guinea pig PRL showed somewhat limited amino acid sequence identity with PRLs from other mammalian species (46–58%; Table 1 and Fig. 1). Greatest amino acid sequence identity (57–58%) was observed with PRLs from the horse, rabbit, squirrel, opossum, and pig (Table 1 and Fig. 1). Maximal disparity between the guinea pig and porcine PRL sequences was associated with exons 3 and 4 of the respective genes and the amino acids they encode.
Figure 1.
Analysis of the predicted amino acid sequence of guinea pig PRL. A, Predicted amino acid sequences for guinea pig (C. porcellus) PRL and porcine (Sus scrofa) PRL. GenBank accession numbers are provided in Table 1. An arrow indicates the predicted signal peptide cleavage sites. The identity of these putative cleavage sites is based on the SignalP software program and similarities with other members of the PRL family. Note similarities in the positioning of cysteines residues (shown as shaded boxes). Asterisks below the sequences denote identity, colons denote strong similarity, and periods denote weak similarity. B, Phylogenetic analysis of PRL in the guinea pig (C. porcellus), horse (Equus cabalas), rabbit (Oryctolagus cuniculus), squirrel (Spermophilus tridecemlineatus), pig (Sus scrofa), opossum (Monodelphis domestica), cat (Felis catus), rhesus monkey (Macaca mulatta), human (Homo sapiens), chicken (Gallus gallus), cow (Bos taurus), sheep (Ovis aries), rat (Rattus norvegicus), golden hamster (Mesocricetus auratus), Japanese grass vole (Microtus montebelli), mouse (Mus musculus), pipid frog (Xenopus tropicalis), African clawed frog (Xenopus laevis), dog (Canis familiaris), Atlantic salmon (Salmo salar), fugu fish (Takifugu rubripes), rainbow trout (Oncorhynchus mykiss), goldfish (Carassius auratus), African tilapia (Oreochromis niloticus), and zebrafish (Danio rerio). Multiple amino acid sequence alignments and phylogenetic tree construction were performed using the CLUSTAL X and TREEVIEW software programs (28,29). Bootstrap values are included within the phylogenetic tree. GenBank accession numbers are provided in Table 1.
Table 1.
PRL sequence analysis
| Genus species | Common name | Accession no. | Amino acids | Percent identity with C. porcellus | Identity with Sus scrofa (%) |
|---|---|---|---|---|---|
| C. porcellus | Guinea pig | GU929698 | 224 | 100 | 57 |
| Equus cabalas | Horse | NP_001075365 | 229 | 58 | 92 |
| Oryctolagus cuniculus | Rabbit | NP_001076144 | 227 | 58 | 88 |
| Spermophilus tridecemlineatus | Squirrel | ENSSTOP00000013231 | 226 | 58 | 89 |
| Sus scrofa | Pig | NP_999091 | 229 | 57 | 100 |
| Monodelphis domestica | Opossum | NP_001028166 | 228 | 57 | 83 |
| Felis catus | Cat | NP_001036806 | 229 | 57 | 91 |
| Macaca mulatta | Rhesus monkey | NP_001040593 | 227 | 55 | 75 |
| Homo sapiens | Human | NP_000939 | 227 | 55 | 77 |
| Gallus gallus | Chicken | NP_990797 | 229 | 51 | 73 |
| Bos taurus | Cow | NP_776378 | 229 | 50 | 79 |
| Ovis aries | Sheep | NP_001009306 | 240 | 50 | 78 |
| Rattus norvegicus | Rat | NP_036761 | 226 | 48 | 61 |
| Mesocricetus auratus | Golden hamster | AAB20367 | 226 | 47 | 57 |
| Microtus montebelli | Japanese grass vole | AAD53180 | 225 | 46 | 61 |
| Mus musculus | Mouse | NP_035294 | 228 | 46 | 62 |
| Xenopus tropicalis | Pipid frog | NP_001093699 | 230 | 45 | 68 |
| Xenopus laevis | African clawed frog | NP_001086486 | 230 | 44 | 66 |
| Canis familiaris | Dog | AAV63936 | 239 | 39 | 57 |
| Salmo salar | Atlantic salmon | NP_001117140 | 210 | 31 | 29 |
| Takifugu rubripes | Fugu fish | NP_001072092 | 213 | 31 | 29 |
| Oncorhynchus mykiss | Rainbow trout | NP_001118205 | 210 | 31 | 29 |
| Carassius auratus | Goldfish | AAB47155 | 210 | 30 | 30 |
| Oreochromis niloticus | African tilapia | CAA00720 | 212 | 29 | 29 |
| Danio rerio | Zebrafish | NP_852102 | 210 | 28 | 29 |
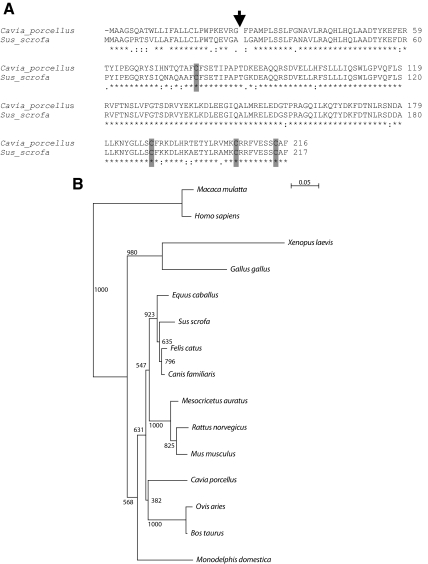
Guinea pig Gh is a five-exon gene similar in structure to other vertebrate Gh genes (7) and spans 1,478 nucleotides on the minus strand of guinea pig.scaffold_65 from nucleotide 5,007,797 to 5,009,255. No additional Gh-related genes were identified in the guinea pig genome. The cDNA for guinea pig Gh was cloned by RT-PCR from guinea pig pituitaries, sequenced, and compared with the previously reported guinea pig Gh sequence (GenBank nucleotide accession no. AF233853) and other vertebrate Gh sequences. The guinea pig Gh cDNA encodes a predicted protein of 216 amino acids with a 26-amino acid signal peptide and in the mature protein possesses cysteine residues in locations homologous to the four cysteines found in other GH proteins (Fig. 2). Unlike guinea pig PRL, guinea pig GH amino acid sequences were highly similar to GHs from other eutherian mammals (84–89% identity; Supplemental Table 2 and Fig. 2).
Figure 2.
Analysis of the predicted amino acid sequence of guinea pig GH. A, Predicted amino acid sequences for guinea pig (C. porcellus) GH and porcine (Sus scrofa) GH. GenBank accession numbers are provided in Supplemental Table 2. An arrow indicates the predicted signal peptide cleavage sites. The identity of these putative cleavage sites is based on the SignalP software program and similarities with other members of the GH family. Note similarities in the positioning of cysteines residues (shown as shaded boxes). Asterisks below the sequences denote identity, colons denote strong similarity, and periods denote weak similarity. B, Phylogenetic analysis of GH in the guinea pig (C. porcellus), dog (Canis familiaris), cat (Felis catus), pig (Sus scrofa), horse (Equus cabalas), golden hamster (Mesocricetus auratus), cow (Bos taurus), rat (Rattus norvegicus), sheep (Ovis aries), mouse (Mus musculus), opossum (Monodelphis domestica), chicken (Gallus gallus), rhesus monkey (Macaca mulatta), human (Homo sapiens), and African clawed frog (Xenopus laevis). Multiple amino acid sequence alignments and phylogenetic tree construction were performed using the CLUSTAL X and TREEVIEW software programs (28,29). Bootstrap values are included within the phylogenetic tree. GenBank accession numbers are provided in Supplemental Table 2.
In summary, guinea pig Prl and Gh genes possess the basic structure characteristic of all PRL and GH family genes; however, these two guinea pig genes have exhibited different rates of evolution. Guinea pig GH shows a high level of conservation with other mammalian GHs, whereas guinea pig PRL is more divergent in structure.
Distribution of PRL and GH
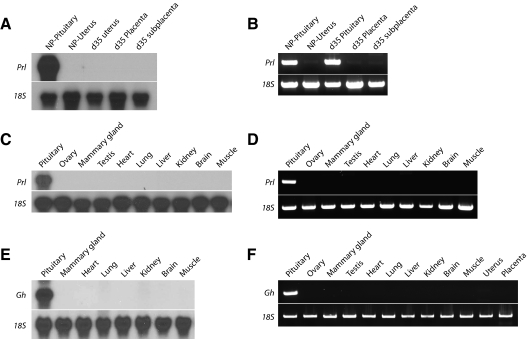
Northern blotting and RT-PCR analyses were used to monitor tissue-specific expression of guinea pig Prl and Gh. Prl and Gh mRNAs were readily detectable by northern blotting in the pituitary (Fig. 3). Transcripts for Prl and Gh were not detectable in uterus, decidua, placenta, ovary, mammary gland, testis, heart, lung, liver, kidney, brain, or muscle (Fig. 3 and Supplemental Fig. 1). PRL and GH proteins were localized to the guinea pig anterior pituitary by immunocytochemistry (Supplemental Fig. 2). In summary, guinea pig PRL and GH are restricted in their expression to the anterior pituitary.
Figure 3.
Tissue distribution of guinea pig Prl and Gh mRNAs. A, Northern blot analysis for Prl mRNA in the pituitary gland and uterus of a cycling female guinea pig (NP) and in the uterus, placenta, and subplacenta from gestation d 35 pregnant guinea pigs. B, RT-PCR analysis for Prl mRNA in the pituitary gland and uterus of a cycling female guinea pig (NP) and in the uterus, pituitary, placenta, and subplacenta of gestation d 35 pregnant guinea pigs. C, Northern blot analysis for Prl mRNA in pituitary, ovary, mammary gland, testis, heart, lung, liver, kidney, brain, and muscle of gestation d 35 pregnant guinea pigs. D, RT-PCR analysis for Prl mRNA in the same tissues shown in C. E, Northern blot analysis for Gh mRNA in pituitary gland, mammary gland, heart, lung, liver, kidney, brain, and muscle. F, RT-PCR analysis for Gh mRNA in pituitary gland, ovary, mammary gland, testis, heart, lung, liver, kidney, brain, muscle, uterus, and placenta. Expression of 18S RNA was used as an internal control for RNA integrity. Note that guinea pig Prl and Gh mRNAs were exclusively expressed in pituitary gland.
Identification and characterization of guinea pig Prlrp1 and Prlrp2 cDNAs
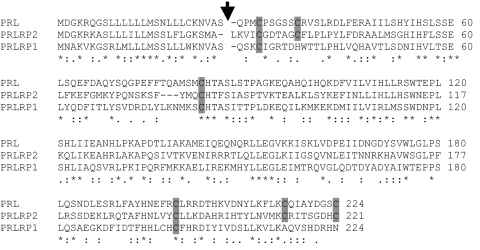
Interrogation of the public guinea pig genome assembly with cDNA sequences for members of the PRL family resulted in the identification of six additional nucleotide sequences more distantly related to Prl. They were situated on guinea pig.scaffold_207, _13, _11, _108, _18, and _123. We characterized two of the sequences possessing the highest homology with Prl, which were termed Prlrp1 and Prlrp2. Prlrp1 and Prlp2 each have a five-exon gene similar to the organization of Prl genes (7). The Prlrp1 gene spans 8,554 nucleotides on the minus strand of guinea pig.scaffold_207 from nucleotide 469,447 to 478,001, whereas the Prlrp2 gene spans 8,457 nucleotides on the minus strand of guinea pig. scaffold_13 from nucleotide 22,425,555 to 22,434,012. Prlrp1 and Prlrp2 cDNAs were cloned by RT-PCR from guinea pig placentas, sequenced, and analyzed by BLAST analysis. Prlrp1 (GenBank accession no. GU929700) encodes a predicted protein of 224 amino acids, with a 26-amino acid signal peptide, and the mature protein possesses four cysteine residues; three are found in locations homologous to the cysteines found in other PRL proteins (Fig. 4). Prlrp2 (GenBank accession no. GU929699) encodes a predicted protein of 221 amino acids, with a 25-amino acid signal peptide, and the mature protein possesses cysteine residues in locations homologous to the six cysteines found in other PRL proteins (Fig. 4). PRLRP1 and PRLRP2 showed limited amino acid sequence identity with sequences from other mammalian species. Greatest amino acid sequence identities were observed with guinea pig PRL (PRLRP1 vs. PRL, 42%; PRLRP2 vs. PRL, 44%) and with each other (PRLRP1 vs. PRLRP2, 37%; Fig. 4). Limited sequence identities were found with members of other mammalian PRL family expansions.
Figure 4.
Analysis of the predicted amino acid sequences of guinea pig PRLRP1 and PRLRP2. Predicted amino acid sequences for guinea pig PRL (GenBank accession no. GU929698), PRLRP1 (GenBank accession no. GU929700), and PRLRP2 (GenBank accession no. GU929699). An arrow indicates the predicted signal peptide cleavage sites. The identity of these putative cleavage sites is based on the SignalP software program and similarities with other members of the PRL family. Note similarities in the positioning of cysteines residues (shown as shaded boxes). Asterisks below the sequences denote identity, colons denote strong similarity, and periods denote weak similarity.
In summary, guinea pig Prlrp1 and Prlrp2 genes possess elements of the basic structure characteristic of all PRL family genes.
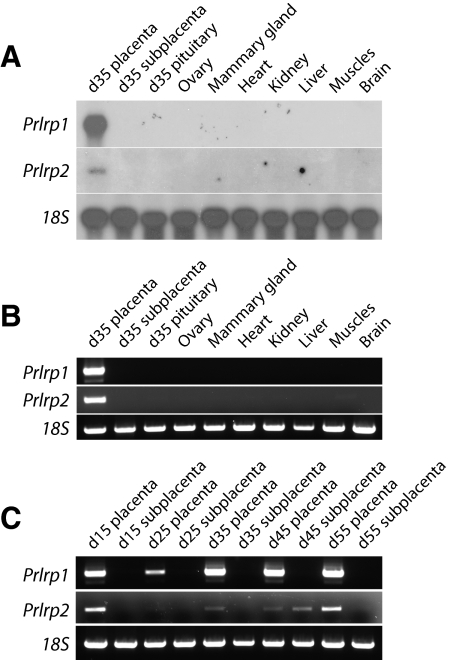
Distribution of Prlrp1 and Prlrp2 transcripts
Northern blotting and RT-PCR analyses were used to monitor tissue-specific expression of guinea pig Prlrp1 and Prlrp2. Prlrp1 and Prlrp2 mRNAs were detectable in the placenta (Fig. 5A). Transcripts for Prlrp1 were not measurable in the subplacenta, anterior pituitary, ovary, mammary gland, testis, heart, lung, liver, kidney, muscle, or brain (Fig. 5, A and B). Prlrp1 mRNA expression increased as gestation progressed (Fig. 5C). In addition to the placenta, Prlrp2 mRNA could be detected in the subplacenta on d 35 of gestation but was not demonstrable in any other tissue (Fig. 5). Overall, Prlrp1 transcript levels were placenta specific and more abundant and easier to detect than transcripts for Prlrp2 (Fig. 5C).
Figure 5.
Tissue distribution of guinea pig Prlrp1 and Prlrp2 mRNAs. A, Northern blot analysis for Prlrp1 and Prlrp2 mRNAs in the placenta, subplacenta, anterior pituitary, ovary, mammary gland, heart, kidney, liver, muscle, and brain from gestation d 35 pregnant guinea pigs. B, RT-PCR analysis of Prlrp1 and Prlrp2 mRNAs in the same tissues shown in A. C, RT-PCR analysis for Prlrp1 and Prlrp2 mRNAs from guinea pig placental and subplacental tissues representing d 15–55 of gestation. Expression of 18S RNA was used as an internal control for RNA integrity. Note that guinea pig Prlrp1 and Prlrp2 mRNAs are expressed in placenta.
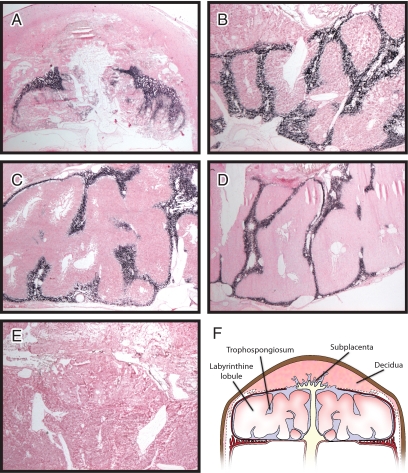
The relative abundance of Prlrp1 prompted an assessment of its localization within the placenta. The guinea pig placentation site is organized into distinct compartments (35,36,37). The main body of the placenta is arranged into labyrinthine lobules in which maternal-fetal exchange takes place. These lobules are separated by interlobial structures (called trophospongium or interlobium). Invasive trophoblast situated at the maternal interface is organized into a structure termed the subplacenta. Prlrp1 mRNA was localized to the trophospongium of the guinea pig placenta and not detected in the labyrinthine lobules or within the subplacenta (Fig. 6). The intraplacental distribution of Prlrp1 transcripts was similar on d 25, 35, 45, and 55 of gestation (Fig. 6).
Figure 6.
In situ localization of Prlrp1 mRNA in guinea pig placentation sites. Prlrp1 transcripts were identified in tissue sections of placentation sites prepared from d 25 (A), 35 (B), 45 (C), and 55 (D) of gestation. Prlrp1 sense and antisense digoxigenin-labeled riboprobes were used in the hybridization analysis. Prlrp1 antisense riboprobes specifically hybridized to the trophospongium structures (dark purple/black staining) separating the labyrinthine lobules. Prlrp1 sense riboprobes did not show specific hybridization (E). Tissue sections were counterstained with nuclear fast red. Organization of the guinea pig placentation site is shown in a schematic (F).
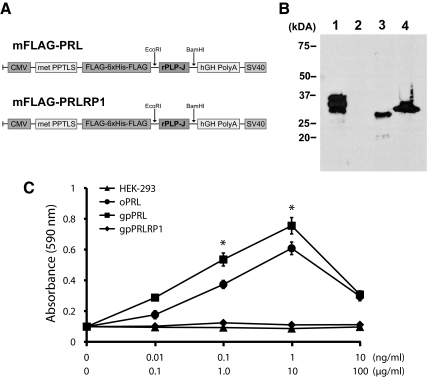
Characterization of guinea pig PRL and PRLRP1 proteins
Recombinant guinea pig PRL and PRLRP1 were produced in a mammalian cell expression system (Fig. 7A). Recombinant FLAG-tagged guinea pig PRL and PRLRP1 proteins migrated as 28- to 30-kDa proteins (Fig. 7B). Biological activities of guinea pig PRL and PRLRP1 were assessed with the Nb2 lymphoma cell proliferation assay, an in vitro assay measuring PRL-like bioactivities (33). Guinea pig PRL significantly stimulated the proliferation of Nb2 lymphoma cells, as did ovine PRL, whereas PRLRP1 and HEK-293 mock-transfected conditioned medium were ineffective (Fig. 7C).
Figure 7.
Effects of guinea pig PRL and PRLRP1 on proliferation of Nb2 lymphoma cells. A, Guinea pig PRL and PRLRP1 were expressed as FLAG-tagged proteins in a HEK-293 cell expression system. B, Western blot analysis of lysates from genetically engineered HEK-293 cells expressing guinea pig PRL (lane 3) or PRLRP1 (lane 4) resolved in 12% sodium dodecyl sulfate-polyacrylamide gels under reducing conditions and probed with anti-FLAG M2 antibodies. Flag-tagged rat PLP-J (lane 1) and conditioned medium from mock-transfected HEK-293 cells (lane 2) were used as positive and negative controls, respectively. C, Quiescent Nb2 cells were incubated for 72 h with conditioned medium from HEK-293 cells genetically engineered to express guinea pig PRL or PRLRP1. Purified ovine PRL (0.1–10 ng/ml) and conditioned medium from mock-transfected HEK-293 cells were used as positive and negative controls, respectively. Cell numbers were assessed with a nonradioactive cell proliferation assay. Values represent the mean ± sd of six replicates. Asterisks denote significant stimulation above negative control values (P < 0.01). Note that guinea pig PRL stimulates the proliferation of Nb2 lymphoma cells.
Thus, guinea pig PRL protein exhibits an expression pattern and biological activity similar to those of PRLs from other species. PRLRP1 is a placental protein with putative actions independent of the canonical PRL signaling pathway. Prlrp2 is a low abundant placental transcript of unknown function.
Discussion
The foundation of modern endocrinology and reproductive biology research was based on experimentation with animal models, including the guinea pig (38). Over the past several decades, the guinea pig lost some of its luster due to advances in the development of genetic tools for other more tractable animal models such as the mouse and more recently the rat. The recent extension of genome sequencing projects to additional species, including the guinea pig, will enable the use of potentially more appropriate animal models to pursue fundamental scientific questions in endocrinology and reproduction. Prime among these new opportunities is the use of the guinea pig for research on the endocrinology of pregnancy, the placenta, and fetal development. The guinea pig shares with humans a hemomonochorial type of placentation and a homologous process of trophoblast invasion and uterine spiral artery remodeling, thus making it an ideal animal model for pregnancy research (35,36,37,39,40,41,42).
PRL is a multifunctional hormone with known biological roles associated with pregnancy. It has been implicated in the regulation of corpus luteum function, mammary gland development, and as a modulator of pregnancy-dependent changes in brain function, pancreas biology, and the immune system (5,43,44,45,46,47). The role of PRL in the biology of pregnancy in the guinea pig has not been evaluated.
In this report, PRL-like nucleotide sequences were first identified in a search of the public guinea pig genome database, which led to isolation of the guinea pig PRL cDNA and protein and characterization of its expression pattern and biological activity. Although the primary structure of guinea pig PRL deviates from other eutherian mammals, which may have hindered earlier attempts at its isolation, its expression in the anterior pituitary gland and its stimulation of Nb2 lymphoma cell proliferation are consistent with its moniker. Unlike other species, extrapituitary expression of PRL was not evident in the guinea pig (48). Our inability to detect PRL in an assortment of tissues may be a consequence of the sensitivities of the assays used and/or the physiological state of the animals sampled. Guinea pig PRL was biologically active as determined with an in vitro cell model system. Such information indicates that guinea pig PRL can interact and activate a canonical PRL signaling pathway; however, the full breadth of actions for guinea pig PRL is yet to be defined. Thus, the significance and evolutionary pressures that drove changes in the primary structure of guinea pig PRL are unknown. This is in contrast to the related anterior pituitary hormone, GH, which exhibits a high level of conservation.
The guinea pig, similar to other rodents (mouse, rat, hamster, deer mouse) and ruminants (cow, sheep, and goat), underwent an expansion at its Prl gene locus (16,17,18,19,20,21,22,49). Examination of the guinea pig genome database did not yield any evidence for an expansion of the Gh locus. An expanded Gh locus is a feature of many primate species (50). The breadth of the guinea pig PRL family expansion appears more limited than found in other species (about seven members); however, this could change up or down as characterization of the Prl locus is finalized. The nature of the guinea pig PRL family expansion is unique and other than the Prl gene exhibits limited similarity to the distinct murine rodent and ruminant PRL family expansions. Orthologs for PRLRP1 and PRLRP2 cannot be readily identified. Similar to PRL family expansions in other species, pregnancy and the placenta appear to be responsible for the selective pressure driving the guinea pig PRL family expansion. Some limited bioassay evidence has suggested the existence of a placental lactogen in the guinea pig (51). This activity is not associated with guinea pig PRL because it is not produced in the placenta. Among the remaining guinea pig PRL family members, both PRLRP1 and PRLRP2 are expressed in the placenta. The more abundant PRLRP1 does not appear to be responsible for the PRL-like bioactivity of the guinea pig placenta. The ineffectiveness of PRLRP1 as an agonist for the canonical PRL signaling pathway may be associated with some of its structural oddities, including limited conservation of the cysteine backbone. Whether the less abundant PRLRP2 or possibly another guinea pig PRL family ligand contributes to PRL-like bioactivities of the guinea pig placenta remains to be determined.
PRLRP1 is a putative placental hormone/cytokine. Its expression persisted throughout gestation and was attributed to the activities of the trophospongium. This region of the guinea pig placenta is comprised of trophoblast syncytia that are bathed by maternal blood and regulate nutrient delivery to the fetal vasculature of the labyrinthine lobules (35,36,37). PRLRP1 is a member of the PRL family with presumed noncanonical actions (independent of the PRL receptor signaling pathway). The junctional zone of the murine rodent placenta is located at the maternal interface and produces several noncanonical PRL family ligands possessing a wide range of biological actions targeted to uterine stromal, vasculature, hematopoietic, and inflammatory cells (16). It has been hypothesized that these noncanonical PRL family ligands contribute to pregnancy-dependent adaptations, improving pregnancy success and survival of the species (16,52,53). The biology associated with PRLRP1 is not yet resolved.
In summary, the isolation and characterization of members of the guinea pig PRL family have yielded a new set of tools and strategies for studying the biology of pregnancy. Furthermore, we have provided a template for expanding the research armamentarium for a neglected animal model such as the guinea pig.
Supplementary Material
Acknowledgments
We thank Dr. Peter Gout (University of British Columbia, Vancouver, BC, Canada) for providing the Nb2 lymphoma cells.
Footnotes
This work was supported by grants from the National Institutes of Health (HD20676, HD39878, HD48861, HD49503) and the Hall Family Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: dT, Deoxythymidine; HEK, human embryonic kidney; PRL, prolactin; PRLRP, PRL-related protein; RACE, rapid amplification of cDNA ends.
References
- Nicoll CS, Bern HA 1972 On the actions of prolactin among the vertebrates: is there a common denominator? In: Wolstenholme GEW, Knight J, eds. Lactogenic Hormones. London: Churchill Livingstone; 299–337 [Google Scholar]
- Nicoll CS 1980 Ontogeny and evolution of prolactin’s functions. Fed Proc 39:2563–2566 [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA 1998 Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA 2002 Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67 [DOI] [PubMed] [Google Scholar]
- Wallis M 2000 Episodic evolution of protein hormones: molecular evolution of pituitary prolactin. J Mol Evol 50:465–473 [DOI] [PubMed] [Google Scholar]
- Forsyth IA, Wallis M 2002 Growth hormone and prolactin—molecular and functional evolution. J Mammary Gland Biol Neoplasia 7:291–312 [DOI] [PubMed] [Google Scholar]
- Sherman GB, Heilman DF, Hoss AJ, Bunick D, Lund LA 2001 Messenger RNAs encoding the β subunits of guinea pig (Cavia porcellus) luteinizing hormone (gpLH) and putative chorionic gonadotropin (gpCG) are transcribed from a single-copy gpLH/CGβ gene. J Mol Endocrinol 26:267–280 [DOI] [PubMed] [Google Scholar]
- Suzuki O, Mochida K, Yamamoto Y, Noguchi Y, Takano K, Matsuda J, Ogura A 2002 Comparison of glycoprotein hormone α-subunits of laboratory animals. Mol Reprod Dev 62:335–342 [DOI] [PubMed] [Google Scholar]
- Keightley MC, Funder JW, Fuller PJ 1991 Molecular cloning and sequencing of a guinea-pig pro-opiomelanocortin cDNA. Mol Cell Endocrinol 82:89–98 [DOI] [PubMed] [Google Scholar]
- Adkins RM, Vandeberg J, Li WH 2000 Molecular evolution of growth hormone and receptor in the guinea-pig, a mammal unresponsive to growth hormone. Gene 246:357–363 [DOI] [PubMed] [Google Scholar]
- Nicoll CS, Bern HA, Brown D 1966 Occurrence of mammotrophic activity (prolactin) in the vertebrate adenohypophysis. J Endocrinol 34:343–354 [DOI] [PubMed] [Google Scholar]
- Nicoll CS, Nichols Jr CW 1971 Evolutionary biology of prolactins and somatotropins. I. Electrophoretic comparison of tetrapod prolactins. Gen Comp Endocrinol 17:300–310 [DOI] [PubMed] [Google Scholar]
- Bethea CL, Hess DL, Widmann AA, Henningfeld JM 1995 Effects of progesterone on prolactin, hypothalamic β-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendocrinology 61:695–703 [DOI] [PubMed] [Google Scholar]
- Vodian MA 1970 Distribution of prolactin and growth hormone in rat and guinea pig adenohypophyses. Life Sci I 9:443–449 [DOI] [PubMed] [Google Scholar]
- Soares MJ, Konno T, Alam SM 2007 The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol Metab 18:114–121 [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Shao LJ, Ain R, Dai G, Soares MJ 2003 The mouse prolactin family locus. Endocrinology 144:313–325 [DOI] [PubMed] [Google Scholar]
- Alam SM, Ain R, Konno T, Ho-Chen JK, Soares MJ 2006 The rat prolactin gene family locus: species-specific gene family expansion. Mamm Genome 17:858–877 [DOI] [PubMed] [Google Scholar]
- Anthony RV, Liang R, Kayl EP, Pratt SL 1995 The growth hormone/prolactin gene family in ruminant placentae. J Reprod Fertil Suppl 49:83–95 [PubMed] [Google Scholar]
- Ushizawa K, Hashizume K 2006 Biology of the prolactin family in bovine placenta. II. Bovine prolactin-related proteins: their expression, structure and proposed roles. Anim Sci J 77:18–27 [Google Scholar]
- Ushizawa K, Takahashi T, Hosoe M, Ohkoshi K, Hashizume K 2007 Expression and characterization of novel ovine orthologs of bovine placental prolactin-related proteins. BMC Mol Biol 8:95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushizawa K, Takahashi T, Hosoe M, Kizaki K, Abe Y, Sasada H, Sato E, Hashizume K 2007 Gene expression profiles of novel caprine placental prolactin-related proteins similar to bovine placental prolactin-related proteins. BMC Dev Biol 7:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke NE, Liebhaber SA 1995 Molecular biology of the growth hormone-prolactin gene system. Vitam Horm 50:385–459 [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas 3rd EJ, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, DeJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin CW, et al. 2005 Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803–819 [DOI] [PubMed] [Google Scholar]
- Dong Y, Thompson LP 2006 Differential expression of endothelial nitric oxide synthase in coronary and cardiac tissue in hypoxic fetal guinea pig hearts. J Soc Gynecol Investig 13:483–490 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG 2007 Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- Page RD 1996 TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358 [DOI] [PubMed] [Google Scholar]
- Faria TN, Deb S, Kwok SC, Talamantes F, Soares MJ 1990 Ontogeny of placental lactogen-I and placental lactogen-II expression in the developing rat placenta. Dev Biol 141:279–291 [DOI] [PubMed] [Google Scholar]
- Ain R, Canham LN, Soares MJ 2003 Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 260:176–190 [DOI] [PubMed] [Google Scholar]
- Alam SM, Konno T, Sahgal N, Lu L, Soares MJ 2008 Decidual cells produce a heparin-binding prolactin family cytokine with putative intrauterine regulatory actions. J Biol Chem 283:18957–18968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Shiu RP, Gout PW, Beer CT, Noble RL, Friesen HG 1980 A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab 51:1058–1063 [DOI] [PubMed] [Google Scholar]
- Heiberger RM, Holland B 2004 Statistical analysis and data display: an intermediate course with examples in S-Plus, R, and SAS. New York: Springer [Google Scholar]
- Kaufmann P, Davidoff M 1977 The guinea-pig placenta. Adv Anat Embryol Cell Biol 53:5–91 [DOI] [PubMed] [Google Scholar]
- Mess A, Zaki N, Kadyrov M, Korr H, Kaufmann P 2007 Caviomorph placentation as a model for trophoblast invasion. Placenta 28:1234–1238 [DOI] [PubMed] [Google Scholar]
- Mess A 2007 The guinea pig placenta: model of placental growth dynamics. Placenta 28:812–815 [DOI] [PubMed] [Google Scholar]
- Keightley MC, Fuller PJ 1996 Anomalies in the endocrine axes of the guinea pig: relevance to human physiology and disease. Endocr Rev 17:30–44 [DOI] [PubMed] [Google Scholar]
- Leiser R, Kaufmann P 1994 Placental structure: in a comparative aspect. Exp Clin Endocrinol 102:122–134 [DOI] [PubMed] [Google Scholar]
- Enders AC 2000 Trophoblast-uterine interactions in the first days of implantation: models for the study of implantation events in the human. Semin Reprod Med 18:255–263 [DOI] [PubMed] [Google Scholar]
- Carter AM 2007 Animal models of human placentation—a review. Placenta 28(Suppl A):S41–S47 [DOI] [PubMed] [Google Scholar]
- Roberts CT, Owens JA, Sferruzzi-Perri AN 2008 Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs. Placenta 29(Suppl A):S42–S47 [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC 1997 Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29:301–307 [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Horseman ND 2001 Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays 23:288–294 [DOI] [PubMed] [Google Scholar]
- Bachelot A, Binart N 2007 Reproductive role of prolactin. Reproduction 133:361–369 [DOI] [PubMed] [Google Scholar]
- Grattan DR, Kokay IC 2008 Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol 20:752–763 [DOI] [PubMed] [Google Scholar]
- Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ 2008 Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia 13:13–28 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW 1996 Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev 17:639–669 [DOI] [PubMed] [Google Scholar]
- Soares MJ 2004 The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2:51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papper Z, Jameson NM, Romero R, Weckle AL, Mittal P, Benirschke K, Santolaya-Forgas J, Uddin M, Haig D, Goodman M, Wildman DE 2009 Ancient origin of placental expression in the growth hormone genes of anthropoid primates. Proc Natl Acad Sci USA 106:17083–17088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Tsushima T, Shiu RP, Friesen HG 1976 Lactogenic and growth hormone-like activities in pregnancy determined by radioreceptor assays. Endocrinology 99:765–774 [DOI] [PubMed] [Google Scholar]
- Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ 2004 A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci USA 101:16543–16548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Konno T, Dai G, Lu L, Wang D, Dunmore JH, Godwin AR, Soares MJ 2007 A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development 134:407–415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.