Abstract
The neuropeptides kisspeptin, neurokinin B, and dynorphin A (collectively abbreviated as KNDy) are, respectively, encoded by KiSS-1, NKB, and PDYN and are coexpressed by neurons of the hypothalamic arcuate nucleus (ARC). Here, using quantitative real-time PCR, we examined age-related changes in the expression of genes encoding KNDy and associated receptors G protein-coupled receptor 54 (encoded by GPR54), neurokinin 3 receptor (encoded by NK3), and κ-opioid receptor (encoded by KOR), in the female rhesus macaque ARC-median eminence (ARC-ME). Expression of KiSS-1 and NKB was highly elevated in old perimenopausal compared with young or middle-aged premenopausal animals. To test whether these age-related changes could be attributed to perimenopausal loss of sex steroids, we then examined KNDy, GPR54, NK3, and KOR expression changes in response to ovariectomy (OVX) and exposure to 17β-estradiol (E2). Short-term (7 months) OVX (with or without 1 month of estrogen replacement) failed to modulate the expression of any of the KNDy-related genes. In contrast, long-term (∼4 yr) OVX significantly increased KiSS-1 and NKB expression, and this was reversed by E2 administration. Finally, we examined the expression of KNDy-related genes in young adult females during the early follicular, late follicular, or midluteal phases of their menstrual cycle but found no difference. Together, the results suggest that short-term alterations in circulating E2 levels, such as those occurring during the menstrual cycle, may have little effect on the ARC-ME expression of KNDy and associated receptors. Nevertheless, they clearly demonstrate that loss of ovarian steroid negative feedback that occurs during perimenopause plays a major role in modulating the activity of KNDy circuits of the aging primate ARC-ME.
Expression of KiSS-1 and NKB, but not PDYN, increased significantly in the female rhesus macaque arcuate-median eminence during aging and after long-term ovariectomy.
Menopause marks the end of reproductive function for women and is characterized by a disruption of the hypothalamic-pituitary-gonadal (HPG) axis. In contrast to rodents, the age-associated decline in circulating sex-steroid hormone concentrations in primates is thought to stem primarily from a depletion of ovarian follicles, rather than from sex-steroid-independent changes within the hypothalamus (1). Even so, hypothalamic changes do occur in perimenopausal women and may have a major impact on the activity of the HPG axis as well as other physiological functions. Currently, there is clear evidence that a population of cells located in the arcuate nucleus (ARC) of the hypothalamus coexpress kisspeptin, neurokinin B, and dynorphin A (2); here, we refer to these neuropeptides collectively by the abbreviation KNDy (2). Importantly, these neurons play a major role in modulating the release of GnRH from the hypothalamus and also show marked changes in their activity after menopause (1). Consequently, it is likely that ARC KNDy neurons serve as conduits for estrogen’s action on GnRH neurons. Although GnRH neurons appear to express estrogen receptor β (3) they do not express estrogen receptor α (ERα) (4,5,6), whereas kisspeptin neurons do (7,8,9,10,11,12,13,14,15); ERα is considered to be the main estrogen receptor type that mediates estrogen negative feedback on GnRH neuronal activity (16,17).
Kisspeptin and neurokinin B appear to be necessary for normal GnRH secretion and reproductive function, because mutations in their receptor genes, G protein-coupled receptor 54 (encoded by GPR54) (18,19) and neurokinin 3 receptor (encoded by NK3) (20), respectively, result in hypogonadotropic hypogonadism. Also, it has been shown in rodents, sheep, and nonhuman primates that a prepubertal rise in GPR54 and KiSS-1 expression leads to the activation of GnRH, which in turn stimulates the pubertal rise of LH release from the anterior pituitary gland (21,22,23,24,25). Kisspeptin and neurokinin B neurons of the ARC appear to exert their influence directly on GnRH neurons via GPR54 and NK3 receptors, respectively, located in the median eminence (ME) (26,27,28), thereby modulating LH release (29,30). Dynorphin A (encoded by PDYN) is also thought to play a role in mediating sex-steroid feedback to GnRH neurons (31), by acting through the κ-opioid receptor (encoded by KOR) to inhibit LH release (32,33,34).
Although expression of KiSS-1, NKB, and PDYN (collectively referred to here as KNDy) in the ARC clearly plays an important role in controlling the HPG axis, its precise function during primate aging is less clear. In rodents, there is evidence that age-related changes within the hypothalamus precede the onset of reproductive senescence (35,36). For example, reduced estrogen-mediated drive from kisspeptin neurons in the anteroventral periventricular nucleus (AVPV) to GnRH neurons appears to be a major factor in age-associated LH-surge dysfunction (37). However, an AVPV kisspeptin neuronal population is not conserved in the sheep, nonhuman primates, or humans, and the primary site of sex-steroid feedback onto GnRH neurons appears to be located within the medial basal hypothalamus (MBH) (38,39,40).
The overall aim of the present study was to gain further insights into the role of hypothalamic KNDy neuropeptides during aging. Using the female rhesus macaque as a translational clinical model, we focused on two specific questions. 1) How does the expression of KNDy and associated receptor-encoding genes change in the ARC-ME during aging? 2) What role do sex steroids play in mediating these age-related changes?
Materials and Methods
Animals
Adult female rhesus macaques (Macaca mulatta) were cared for by the Division of Animal Resources at the Oregon National Primate Research Center (ONPRC) in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. The animals were housed indoors under controlled conditions: 24 C temperature; 12-h light, 12-h dark photoperiods (lights on at 0700 h); regular meals at 0800 and 1500 h (Purina High Protein Monkey Chow; Purina Mills, Inc., St. Louis, MO) supplemented with fresh fruit and vegetables; and fresh drinking water available ad libitum. They were subjected to the following Institutional Animal Care and Use Committee-approved studies, which involved postmortem isolation of RNA from the ARC-ME and quantification of KiSS-1, NKB, PDYN, GPR54, NK3, and KOR expression using real-time PCR.
Experiment 1: effect of age in ovary-intact animals
Postmortem rhesus macaque brain tissue was obtained through the ONPRC Tissue Distribution Program. The ovary-intact females had previously been involved in a cross-sectional primate aging study and comprised three groups: young (13.9 ± 2.1 yr; n = 5); middle-aged premenopause, regular cycling (22.2 ± 0.4 yr; n = 6); and old perimenopausal, irregular cycling (25.8 ± 0.4 yr; n = 4). Serum, collected at time of necropsy, was frozen and later assayed for 17β-estradiol (E2) and progesterone (P4). Mean E2 concentrations were 40 ± 6, 71 ± 35, and 62 ± 16 pg/ml for the young, middle-aged, and old animals, respectively. Based on individual animal menstruation records and very low or undetectable (<0.2–0.8 ng/ml) P4 concentrations, most of the animals were assumed to be in the follicular phase of their menstrual cycle. The one exception was a young animal that showed elevated P4 concentrations (2.4 ng/ml) and so was assumed to be in the midluteal phase; all of the animals in the old age group showed undetectable (<0.2 ng/ml) P4 concentrations.
Experiment 2: effect of short-term E2 treatment in young ovariectomized (OVX) animals
Adult rhesus macaques were bilaterally OVX and approximately 7 months later were assigned to one of following experimental groups: untreated controls (OVX; mean ± sem age, 9.7 ± 0.3 yr; n = 4) or treated with E2 for 1 month (OVX+E2; mean ± sem age, 9.6 ± 0.8 yr; n = 4). Terminal serum E2 concentrations were undetectable in the OVX group and had a mean value of 117 ± 7 pg/ml in the OVX+E2 groups. For reference, the gene expression levels of these two groups were analyzed together with those of the young gonad-intact animals from experiment 1 (13.9 ± 2.1 yr, n = 5 per group), which showed a mean serum E2 concentration of 40 ± 6 pg/ml at necropsy.
Experiment 3: effect of long-term E2 treatment in old OVX animals
Old female macaques were bilaterally OVX and approximately 2.5 months later were assigned to one of the following experimental groups: untreated controls (OVX; mean ± sem age, 25.0 ± 1.7 yr; n = 4) or treated with E2 for about 4 yr (OVX+E2; mean ± sem age, 27.0 ± 0.9 yr; n = 4). Serum E2 concentrations were randomly measured across the study. In the OVX group, E2 concentrations were either undetectable or just above the detection threshold; when an arbitrary value of 20 pg/ml was assigned to the undetectable levels, the mean concentration was 26 ± 2 pg/ml. In contrast, the mean E2 concentration shown by the OVX+E2 group was 124 ± 11 pg/ml. For reference, the gene expression levels of these two groups were analyzed together with those of the old gonad-intact perimenopausal animals from experiment 1 (mean ± sem age, 25.8 ± 0.4 yr; n = 4), which showed a mean serum E2 concentration of 62 ± 16 pg/ml at necropsy.
Experiment 4: effect of menstrual cycle phase
Adult ovary-intact macaques were monitored daily for menstrual cycle regularity, and cycle phase was corroborated with serum assays for E2 and P4. Necropsies were performed at three distinct phases: 1) the early follicular phase (EF) (3.8 ± 0.3 d after the start of the last menses); the animals were 10.5 ± 0.6 yr old (n = 4) and showed a characteristic rise in serum E2 concentrations (65 ± 19 pg/ml) and undetectable (<0.2 ng/ml) P4 concentrations; 2) the late follicular phase (LF) (11.8 ± 1.3 d after the start of the last menses); the animals were 12.2 ± 0.2 yr old (n = 4 per group) and showed declining E2 concentrations (71 ± 43 pg/ml) and the start of a P4 rise (0.5 ± 0.1 ng/ml); and 3) the midluteal phase (ML) (15.8 ± 1.7 d after the start of the last menses); the animals were 11.2 ± 0.4 yr old (n = 4) and showed characteristic low E2 concentrations (39 ± 4 pg/ml) and high P4 concentrations (4.9 ± 0.8 ng/ml). Phases of the menstrual cycle were further corroborated at necropsy by histological examination of the endometrium: EF, thin endometrium and ciliated oviduct; LF, thin, proliferative endometrium and oviduct ciliated and fully secretory; and ML, thickened endometrium (41).
Experiment 5: 24-h changes in gene expression in young OVX animals
To examine whether gene expression differs between the day and night, we compared ARC-ME gene expression at 0100 h (n = 3) with that of 1300 h (n = 4). The animals were young OVX macaques, and before necropsy, they had been maintained under the same 12-h light, 12-h dark photoperiods. During the nighttime tissue collections, the animals’ eyes were covered (to shield from light exposure).
E2 treatment
In experiments 2 and 3, the E2 was administered via sc SILASTIC brand (Dow Corning, Midland, MI) implants, as previously described (42). The resulting circulating hormone concentrations were similar to those typically observed during the late follicular phase of the menstrual cycle. In experiment 3, the implants were replaced after each year of treatment to ensure sustained long-term delivery of the steroid.
Hormone assays
Blood samples were centrifuged and the supernatant serum was stored at −80 C until time of assay. Serum E2 and P4 concentrations were determined by the ONPRC Endocrine Technology and Support Core using a chemiluminescence-based automatic clinical platform (Immulite 2000; Siemens Healthcare Diagnostics, Deerfield, IL). The sensitivity of the E2 and P4 assays was 20 pg/ml and 0.2 ng/ml, respectively. The intra- and interassay coefficients of variation were less than 15% for both assays.
Tissue collection and RNA extraction
Postmortem brain tissue used in this study was obtained through the ONPRC Tissue Distribution Program. The animals were sedated with ketamine (15–25 mg/kg im) followed by pentobarbital sodium (25–30 mg/kg iv), a procedure consistent with the recommendations of the American Veterinary Medical Association’s Panel on Euthanasia. Each brain was flushed with 1 liter of 0.9% saline via a cardiac catheter, and the MBH was removed and preserved in RNAlater (Ambion, Austin, TX) for 2 wk. A coronal slice encompassing the ARC-ME was removed and stored frozen at −80 C. The boundaries for this tissue block included the exterior ventral edge of the ME, lateral cuts midway between the third ventricle and the optic nerve, an anterior cut along the posterior edge of the optic chiasma, a posterior cut just anterior to the mammillary bodies, and a cut 1 mm dorsal to the base of the third ventricle (i.e. based on stereotaxic coordinates, this represents the border between the ARC and the ventromedial hypothalamus). Subsequently, each ARC-ME block was individually homogenized using a PowerGen rotor-stator homogenizer (Fisher Scientific, Pittsburgh, PA), and RNA was extracted using a QIAGEN (Valencia, CA) RNeasy Mini Kit. Integrity of the RNA was verified using spectrophotometry (Agilent 2100 Bioanalyzer Nanodrop; Agilent Technologies, Santa Clara, CA), and acceptable A260/A280 ratios were used for the determination of RNA concentrations. For each sample, 1 μg RNA was then converted to cDNA using random hexamers and the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen Life Technologies, Carlsbad, CA).
Primers and probes
Table 1 depicts the TaqMan primers and probes that were used for detection of the neuropeptide genes (KiSS-1, NKB, and PDYN) and their associated receptor genes (GPR54, NK3, and KOR) as well as for two housekeeping genes (ALG9 and RPL13A), which we previously showed to be stably expressed in the rhesus ARC-ME across various sex-steroid environments (43). The nucleotide sequences for these genes either were obtained from previously reported studies or were designed using Primer Express 2.0 software [Applied Biosystems (ABI), Foster City, CA] and were based on NCBI rhesus macaque reference sequences. The primers and probes were purchased from Invitrogen and Applied Biosystems (Carlsbad, CA), respectively. RT-PCR, using ARC-ME and ovarian RNA samples (as positive controls), was used to validate each of the primer sets by producing amplicons of a predicted size.
Table 1.
TaqMan real-time PCR primers and probes
| Gene name | Primer sequence (5′–3′) | Probe sequence (5′–3′) | GenBank accession ID |
|---|---|---|---|
| KiSS-1 | 6FAM-CAGGCCAGCAGCTGGAATCCCTG-TAMRA | AY823262 | |
| Forward | AGAAAAGGTGGCCTCTGTGGA | ||
| Reverse | AGGCTCTGCTCCCACGG | ||
| NKB | 6FAM-ACAGCAAGAGGGATCT-MGB | XM_001115535 | |
| Forward | GAGCCACAGGAGGAGATGGTT | ||
| Reverse | TCCAGAGATGAGTGGCTTTTGA | ||
| PDYN | 6FAM-CCAGTTCAAGGTGGTGAC-MGB | XM_001113720 | |
| Forward | CAAGCTCAAGTGGGACAACCA | ||
| Reverse | CTCCAGAGTAAGCATTCGGATCTT | ||
| GPR54 | 6FAM-ACAAGCCGATGCGGACCGTGA-TAMRA | AY823261 | |
| Forward | AACTCGCTGGTCATCTACGTCAT | ||
| Reverse | TCTGTGGCCGCCAGGTT | ||
| NK3 | 6FAM-ATGGCTATTATTGATCCCCTGAAACCCAGA-MGB | AB180075 | |
| Forward | CCATTGCGGTGGACAGGTA | ||
| Reverse | TTGGTTGCTGTGGCAGACA | ||
| KOR | 6FAM-TGGTGGAGGCTCTG-MGB | XR_012897 | |
| Forward | GCTGGACCCCCATTCACATA | ||
| Reverse | GCCTAAGGCGATGCAGAAGT | ||
| ALG9 | 6FAM-ACTGTCTTCCTGTTCGGG-MGB | XM_001106180 | |
| Forward | AACAGTGCCACAGAGCGAGAA | ||
| Reverse | CGATACCGCCTGGAGCACTA | ||
| RPL13A | 6FAM-CCAGGCAGTGACAGCCACCTTGG-MGB | XM_001115079 | |
| Forward | TCACGAGGTTGGCTGGAAGT | ||
| Reverse | GATCTTGGCTTTCTCCTTCCTCTT |
Reference sequences for each of the targeted Macaca mulatta genes can be accessed via GenBank accession ID.
TaqMan quantitative RT-PCR
Quantitative real-time PCR data were obtained using a 7900HT Fast Real-Time PCR thermal cycler and sequence detection system software (version 2.2.1; ABI). Initially, pooled cDNA was used to create standard curves for each gene, and the experimental samples were subsequently diluted accordingly so as to fall within the linear part of the curve. The PCR mixtures contained 5 μl TaqMan Universal PCR Master Mix, 0.3 μl of each specific forward and reverse primer (300 nm final concentration), 0.25 μl of specific probe (250 nm final concentration), and 2 μl of cDNA. The reaction sequence included 2 min at 50 C, 10 min at 95 C, and 50 cycles of 15 sec at 95 C and 1 min at 60 C. Automatic baseline and threshold levels were determined by ABI sequence detection system software (version 2.2.1.), and the final expression values are expressed as ratios relative to the average of two reference genes, ALG9 and RPL13A. Individual genes from experiments 1–3 were examined together on the same 384-well optical plate, whereas in experiments 4 and 5, they were examined on separate plates. A negative control included the omission of cDNA templates from the reaction mixture.
Statistical analysis
In experiments 1–4, one-way ANOVA followed with the post hoc Student-Newman-Keuls test was used to analyze differences between group means. In experiment 5, differences between the two groups were analyzed using the Mann Whitney U test. P < 0.05 was considered to be statistically significant.
Results
KiSS-1, NKB, and PDYN
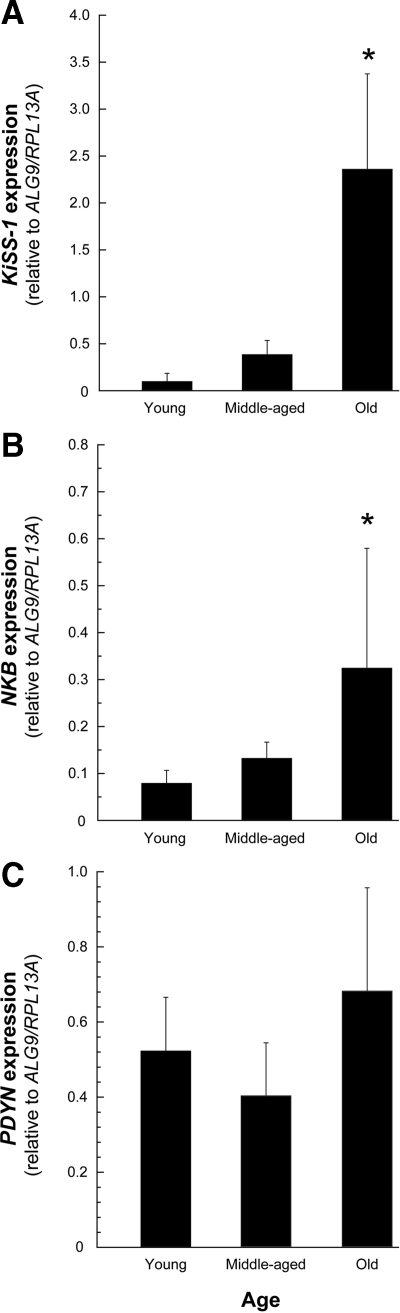
KiSS-1 and NKB expression were significantly elevated (P < 0.05) in old perimenopausal animals compared with the young and middle-aged premenopausal animals (Fig. 1, A and B). PDYN expression levels did not differ between the experimental groups (Fig. 1C).
Figure 1.
Age-related changes in KNDy expression in the ARC-ME of adult female rhesus macaques, revealed by real-time PCR. The values represent the means (±sem) from young (13.9 ± 2.1 yr), middle-aged (22.2 ± 0.4 yr), and old perimenopasual (25.8 ± 0.4 yr) animals. A and B, Expression of KiSS-1 and NKB increased significantly in the old animals relative to the other adults groups; C, PDYN showed no age-related change in expression. *, P < 0.05, ANOVA followed by Student-Newman-Keuls test.
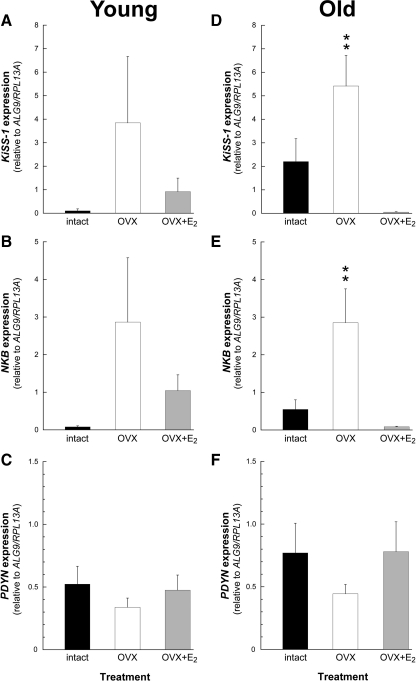
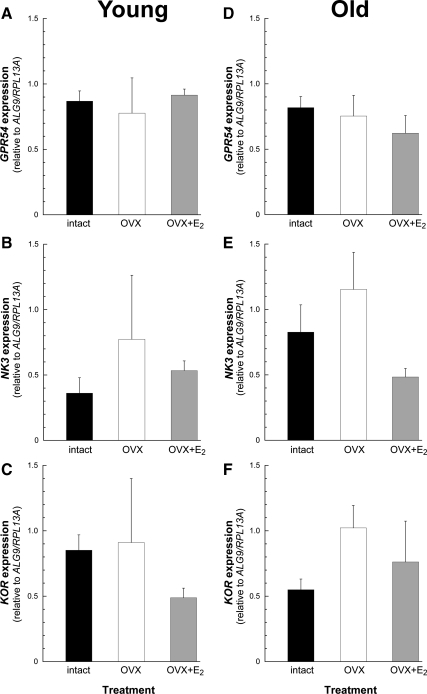
In the young animals (experiment 2), the expression levels of KiSS-1, NKB, or PDYN were not significantly different between the three treatment groups, regardless of gonadal status (ovary-intact vs. OVX) or short-term E2 replacement (OVX+E2) (Fig. 2, A–C). In contrast, in the old animals (experiment 3), the expression levels of KiSS-1 and NKB showed a significant (P < 0.01) increase after long-term OVX, and this was blocked by long-term E2 replacement (OVX+E2) (Fig. 2, D and E). As with the young animals (Fig. 2C), the old animals showed no effect of gonadal status or E2 treatment on PDYN expression (Fig. 2F).
Figure 2.
Effect of short-term (A–C) or long-term (D–F) estrogen replacement on KNDy expression in the ARC-ME of young adult and old female rhesus macaques. The values represent the means (±sem) from ovary-intact (black bars), OVX (white bars), and OVX+E2 (gray bars) animals. In the young age group (A–C), the expression level of KNDy was similar regardless of treatment. In the old animals (D and E), the expression of KiSS-1 and NKB was significantly elevated after OVX and restored to ovary-intact levels by the E2 treatment; the expression of PDYN (F) in the old animals was not different between treatments. **, P < 0.01, ANOVA followed by Student-Newman-Keuls test.
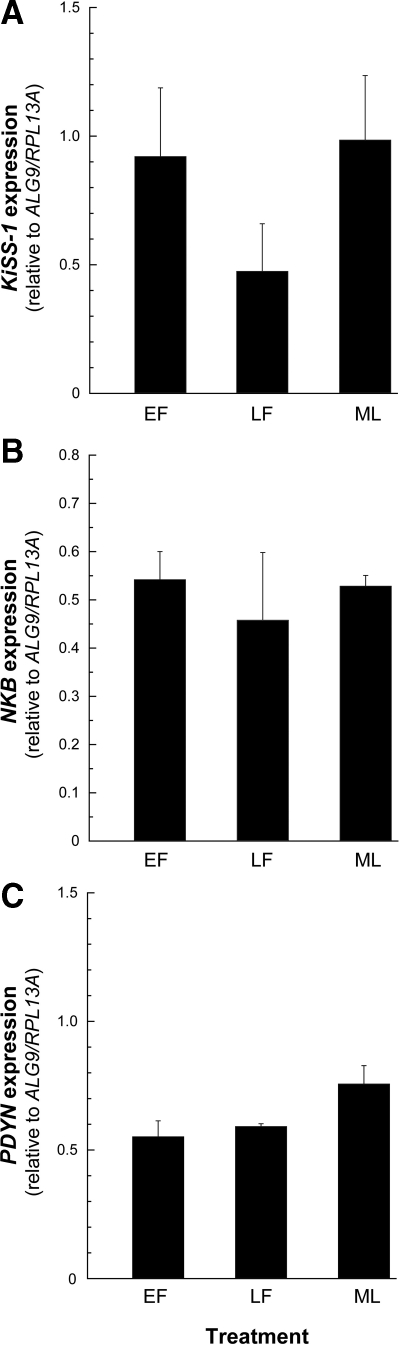
No differences in KiSS-1, NKB, or PDYN expression were observed across the menstrual cycle of young adult animals (Fig. 3, A–C).
Figure 3.
Expression of KNDy in the ARC-ME of adult female rhesus macaques across the menstrual cycle. The values represent the means (±sem) of KiSS-1 (A), NKB (B), and PDYN (C) during the EF, LF, and ML phases of the cycle. No changes in the level of KNDy expression were detected. P > 0.05, ANOVA followed by Student-Newman-Keuls test.
GPR54, NK3, and KOR
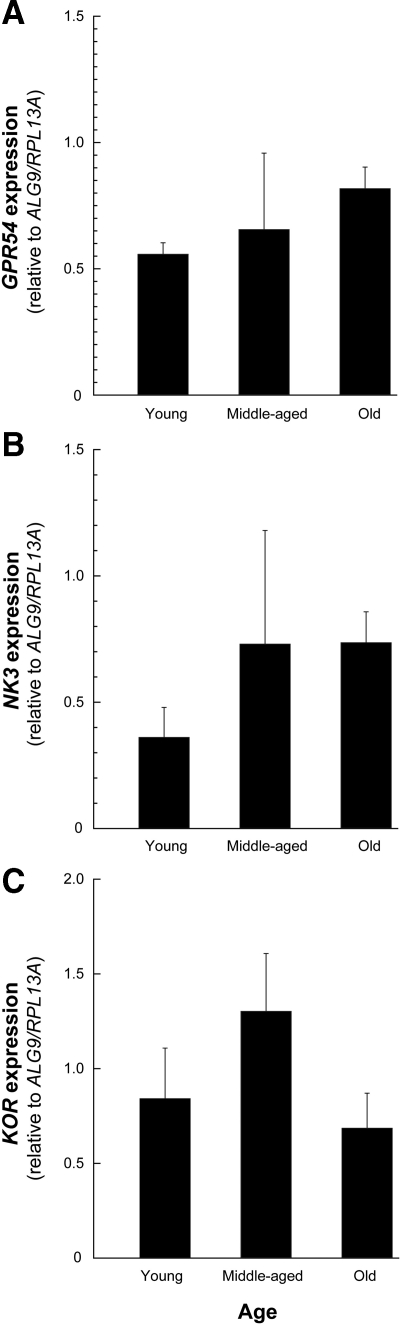
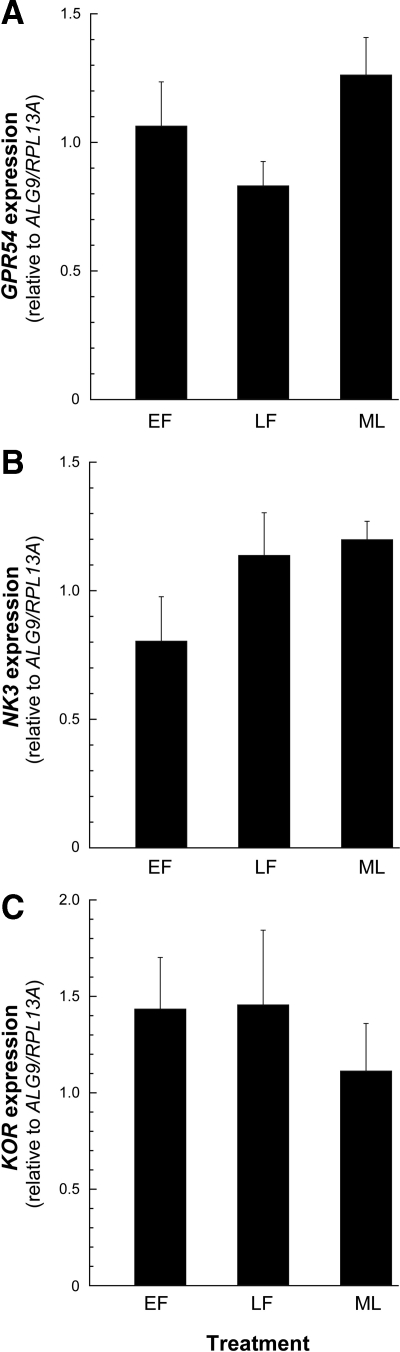
GPR54, NK3, and KOR expression levels were similar in the young, middle-aged premenopausal, and old perimenopausal ovary-intact animals (Fig. 4, A–C). Furthermore, there was no obvious effect of gonadal status or short-term or long-term E2 treatment on the expression of these receptor-encoding genes (Fig. 5, A–F). Similarly, no differences in GPR54, NK3, or KOR expression were observed across the menstrual cycle of young adult animals (Fig. 6, A–C).
Figure 4.
Expression of KNDy receptor genes in the ARC-ME of adult female rhesus macaques during aging. The values represent the means (±sem) of GPR54 (A), NK3 (B), and KOR (C) from young (13.9 ± 2.1 yr), middle-aged (22.2 ± 0.4 yr), and old perimenopausal (25.8 ± 0.4 yr) animals. No changes in the level of GPR54, NK3, or KOR were detected. P > 0.05, ANOVA followed by Student-Newman-Keuls test.
Figure 5.
Effect of short-term (A–C) or long-term (D–F) estrogen replacement on KNDy receptor gene expression in the ARC-ME of young adult and old female rhesus macaques. The values represent the means (±sem) from ovary-intact (black bars), OVX (white bars), and OVX+E2 (gray bars) animals. No changes in the level of GPR54, NK3, or KOR were detected. P > 0.05, ANOVA followed by Student-Newman-Keuls test.
Figure 6.
Expression of KNDy receptor genes in the ARC-ME of adult female rhesus macaques across the menstrual cycle. The values represent the means (±sem) of GPR54 (A), NK3 (B), and KOR (C) during the EF, LF, and ML phases of the cycle. No changes in the level of GPR54, NK3, or KOR were detected. P > 0.05, ANOVA followed by Student-Newman-Keuls test.
Gene expression at 0100 and 1300 h
We considered the possibility that our failure to detect any significant changes in PDYN expression may have stemmed from performing necropsies at a time of day when transcription was at a diurnal low point (44,45), with less obvious between-group differences. As a result, we compared the expression of PDYN as well as KiSS-1, NKB, GPR54, NK3, and KOR at two different times of day (Table 2). Although there was a tendency for some of the genes to show diurnal differences in expression, only GPR54 showed a statistically significant (P < 0.05) difference, with expression levels being significantly higher at 1300 h than at 0100 h (i.e. they were higher at the approximate time of day when all of the necropsies in experiments 1–4 were performed). Importantly, PDYN expression was similar regardless of whether the necropsies were performed in the middle of the day or in the middle of the night.
Table 2.
Diurnal differences in gene expression in the rhesus macaque ARC-ME
| Gene | 0100 h | 1300 h |
|---|---|---|
| KiSS-1 | 0.35 ± 0.18 | 1.58 ± 0.96 |
| NKB | 0.24 ± 0.17 | 1.82 ± 0.75 |
| PDYN | 0.59 ± 0.12 | 0.61 ± 0.28 |
| GPR54 | 0.09 ± 0.01 | 0.40 ± 0.10a |
| NK3 | 0.05 ± 0.04 | 1.25 ± 0.51 |
| KOR | 1.10 ± 0.40 | 0.45 ± 0.13 |
Values represent the mean ± sem from three to four ovariectomized female adults and are expressed relative to the expression of RPL13A.
P < 0.05.
Discussion
Like women, old female rhesus macaques undergo menopause and begin to show a marked decline in circulating E2 concentrations during the perimenopausal period (46). Consequently, they represent a pragmatic animal model in which to examine the neuroendocrine mechanisms associated with human female reproductive aging. The present study shows that the expression of KiSS-1 and NKB in the ARC increases significantly during the perimenopause. This observation is consistent with previous findings that hypothalamic KiSS-1 expression increases in postmenopausal women and macaques (47,48). However, in the present study, we measured serum E2 and P4 concentrations only in a single terminal blood sample and so cannot be sure whether the three different groups had sustained periods of differential serum sex-steroid concentrations; furthermore, based on individual animal menstruation records and very low or undetectable P4 concentrations, all of the animals (except for one) were considered to either be in the early follicular phase of the menstrual cycle or to be acyclic and hence showed a less dramatic age-related decline in serum E2 concentrations. Nevertheless, the data show a clear age-related difference, which plausibly stems from an attenuation of cyclic ovarian steroid concentrations. This possibility was examined in experiment 3, which showed that long-term removal of E2, through OVX, significantly enhances KiSS-1 and NKB gene expression in the ARC-ME. Overall, this finding is in agreement with previous reports that NKB expression increases in postmenopausal women and OVX macaques, resulting in neuronal hypertrophy in the ARC (49,50,51). Importantly, our data also demonstrate that the post-OVX increase in KiSS-1 and NKB expression could be completely blocked by treatment with E2. Furthermore, because the expression of KiSS-1 across the three experimental groups was highly correlated with that of NKB (r2 = 0.95), it is likely that sex-steroid control of these two neuropeptide systems is mediated through a common pathway. Collectively, findings from the present and previously published studies suggest that perimenopausal changes in KiSS-1 and NKB expression stem directly from changes in the sex-steroid environment rather than from aging per se. Consequently, these gene expression changes are likely to be associated with a consequence rather than with a root cause of nonhuman primate and human reproductive senescence.
In contrast to these KiSS-1 and NKB responses shown after long-term OVX and E2 replacement, the responses observed in our short-term, 1-month estrogen treatment model (experiment 2) were less obvious; although there was a similar overall trend and some individuals clearly showed changes similar to those of animals in experiment 3, the results were not statistically significant. It is plausible that larger group sizes would have disclosed more clear-cut between-group differences, but the present results may also suggest that short-term manipulation of the sex-steroid environment is simply too subtle to significantly modulate KiSS-1 and NKB expression in the ARC of young adults. This view is corroborated by our observation that neither gene showed a significant change in expression level when examined at three stages of the menstrual cycle: EF, LF (immediately after the LH surge), and ML phases. Because we failed to observe an increase in ARC KiSS-1 expression during the LF phase, it is tempting to further conclude that kisspeptin plays no role in the kisspeptin (i.e. protein) preovulatory LH surge of primates. However, such a conclusion may not be warranted, especially in view of the recent report of preovulatory elevated Kiss-1 expression in the sheep ARC (52). Although animals in experiment 4 were correctly assigned to the three different phases of the menstrual cycle, based on multiple criteria, including days after menstruation, uterine histology, and serum E2 and P4 concentrations, the serum E2 concentrations of the LF group were not as high as might have been expected. This raises the possibility that some of the animals in this group had already ovulated and were in the descending phase of the preovulatory surge. Consequently, we cannot rule out the possibility that in primates, a transitory increase in KiSS-1 and/or NKB occurs relatively early during the LF phase of the menstrual cycle, before the preovulatory LH surge. Nevertheless, our data emphasize that there is no obvious sustained modulation of KiSS-1 or NKB expression across the natural menstrual cycle, which is in contrast to the marked changes observed during aging and after experimental manipulation of the sex-steroid environment.
A subtle decrease in PDYN expression has recently been observed in the ARC neurons of postmenopausal women (53). Furthermore, there is evidence that PDYN expression decreases after OVX in sheep (54) and that E2 treatment appears to enhance ARC PDYN expression in sheep (54,55). Although we detected a trend for PDYN levels to decrease after OVX (experiments 2 and 3), the results were not statistically significant. It is unclear whether this reflects subtle differences between species or whether our real-time PCR methodology lacked sufficient sensitivity to effectively detect a change. It is also possible that PDYN expression changes are more pronounced at times of the day that were not examined in our study. Using gene microarray analysis, we previously found that many genes have a clear 24-h expression pattern (44,45). Consequently, we were concerned that the collection of postmortem tissue in the present study may have been performed at a suboptimal time of the day (i.e. during the daily nadir of KNDy gene expression) and that this may have hampered our efforts to detect changes in PDYN. Therefore, experiment 5 was designed to compare the expression of ARC genes during the middle of the day to the expression occurring during the middle of the night. Although limited in its scope, the experiment nevertheless failed to disclose any significant diurnal differences in PDYN, suggesting that time of necropsy is unlikely to have been a major factor in our failure to detect changes in expression. It should also be noted that the present study does not rule out the possibility that E2 exerts an influence on ARC dynorphin A neurons at the translational, rather than at the transcriptional, level. Therefore, despite our negative findings, we need to be cautious before concluding that E2 has no regulatory effect on dynorphin A in the primate ARC.
In addition to examining KNDy genes, the present study also comprehensively examined the effects of age and E2 on the ARC-ME expression of KNDy-associated receptor genes. The expression of GPR54, NK3, and KOR was similar in young, middle-aged, and old perimenopausal animals and also did not change after experimental manipulation of the sex-steroid environment or show changes across the menstrual cycle. Hypothalamic GPR54 expression has been reported to increase in postmenopausal macaques but not in young adult animals after OVX (48). These findings, combined with the present results suggest that GPR54 expression in the macaque hypothalamus increases only during very old age and not necessarily in response to declining E2 concentrations. Interestingly, GPR54 was the only gene associated with KNDy neuropetide pathways that showed an obvious diurnal expression pattern. GPR54 levels were significantly higher in the middle of the day (which is when tissues for experiments 1–4 were collected) than in the middle of the night. Consequently, it is plausible that diurnal GPR54 expression plays a role in maintaining normal circadian functions within the hypothalamus and that changes in GPR54 expression after menopause (48) contribute to perturbed circadian rhythms in the elderly. Our finding that GPR54 was more highly expressed in the middle of the animals’ subjective day (i.e. at 1300 h) than in the middle of the subjective night (i.e. at 0100 h) may have implications for studies involving nocturnal species such as rodent. In these studies, postmortem brain tissues are typically collected in the daytime (i.e. during the animals’ subjective night), which may not be the optimal time of day for disclosing changes in Gpr54 expression.
Although the exact role of KNDy neuropeptides in the primate hypothalamus is unclear, there is evidence for its involvement in the control of GnRH release. For example, rodent and sheep studies have shown coexpression of KNDy neuropetides in ARC neurons of the hypothalamus (2,15,55), which is consistent with findings from humans and nonhuman primates (1), and these neurons innervate GnRH fibers in the ME to regulate the secretion of LH (2,24,27,28,31,56). It has also been shown that KNDy neurons coexpress NK3 and KOR in the rodent ARC, which suggests that they may be involved in mediating the effects of estrogen on GnRH pulsatile secretion via autocrine mechanisms (15). In primates, the transition to menopause is associated with increased pulsatile GnRH secretion and LH release (57,58). In addition, E2 effectively suppresses gonadotropin release in postmenopausal women (59), and E2 and P4 treatment attenuates gonadotropin pulse frequency (60). Because ARC-ME KNDy neurons express estrogen and progesterone receptors (7,8,9,10,11,12,13,14,15), and show ERα-mediated negative feedback of estrogen on GnRH (16,17), it is likely that in primates, as in rodents and sheep, these neurons act at the level of GnRH neuronal terminals to mediate steroid negative feedback on GnRH release. Importantly, our data emphasize that this negative feedback loop is likely to be functional even in old perimenopausal animals and thus responsive to hormone replacement therapy.
A small population of kisspeptin neurons resides in the preoptic area and has been implicated in steroid-mediated regulation of gonadotropin secretion in the rodent and sheep (52,61). However, it is unlikely that these neurons play a significant role in modulating gonadotropin release in primates, which appear to rely on neuronal circuits within the MBH for synchronization of LH pulses and for mediation of the positive and negative feedback effects of ovarian hormones (38,39,40,62,63). This conclusion is further supported by a recent study of pre- and postmenopausal rhesus macaques, which reported significant age-related changes in KiSS-1, GPR54, and GnRH-1 expression in the MBH but not in the preoptic area (48).
Although our study focused on age-related loss of estrogen negative feedback and ARC gene expression, many rodent experiments have also examined how positive estrogen feedback is mediated to GnRH neurons via Kiss-1 in the AVPV (37). However, this AVPV kisspeptin neuronal population appears to be unique to rodents, because there is no evidence that it also exists in sheep, nonhuman primates, or in humans. Recently, positive feedback action of estrogen has been observed within ARC Kiss-1 neurons of the sheep during the E2-induced LH-surge (52), but it remains to be determined whether a similar positive feedback mechanism also exists in primates.
In summary, the transition to menopause in nonhuman primates, as in women, is preceded by irregular menstrual cycles and declining levels of circulating E2 (46). The present study further elucidates the role of negative feedback of E2 on KNDy regulation and shows how it is likely to contribute to age-associated neuroendocrine alterations within the hypothalamic ARC-ME. The primary change was a marked increase in KiSS-1 and NKB expression, which was already detectable during the perimenopause, as menstrual cycles become irregular and E2 output from the ovaries starts to decline (46). No corresponding alterations in the expression of KNDy-associated receptor genes were observed, suggesting that the primary modulation of the hypothalamic KNDy pathways is mediated through changes in expression of the ligands rather than the receptors.
Acknowledgments
We thank Dr. Francis Pau of the ONPRC Endocrine Technology Support Core, Yibing Jia and Corey Singleton of the ONPRC Molecular and Cell Biology Core, and Sharon Kryger of the ONPRC Aging Resource for technical assistance. We also thank Dr. Ov Slayden for his help with the uterine histology.
Footnotes
This work was supported by National Institutes of Health Grants T32 AG-023477, R01 AG-029612, U54 HD-018185, R01 HD-029186, and P51 RR-000163.
D.H.E. is now a member of the Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, Oregon.
Disclosure Summary: The authors have nothing to declare.
First Published Online June 2, 2010
Abbreviations: ARC, Arcuate nucleus; AVPV, anteroventral periventricular nucleus; E2, 17β-estradiol; EF, early follicular; HPG, hypothalamic-pituitary-gonadal; KNDy, kisspeptin, neurokinin B, and dynorphin A; LF, late follicular; MBH, medial basal hypothalamus; ME, median eminence; ML, midluteal; P4, progesterone; OVX, ovariectomized.
References
- Rance NE 2009 Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN 2010 The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z 2001 Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW 1983 Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 30:345–347 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT 1992 Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50:283–298 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Caraty A, Allingham R 2001 Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 142:573–579 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of Kiss1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young 3rd WS 1990 Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab 71:79–85 [DOI] [PubMed] [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE 2000 Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- Pillon D, Caraty A, Fabre-Nys C, Bruneau G 2003 Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15:749–753 [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Merchenthaler I 2004 Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A 2006 Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA 2009 Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA 2009 Regulation of GnRH secretion by Kiss1/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE 2003 Critical role for estrogen receptor α in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology 78:204–209 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TAC3R mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB 2005 Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol 26:131–138 [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM 2005 Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E 2008 An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE 2005 Morphological evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM 2008 Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE 2008 Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN 2006 Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- Schulz R, Wilhelm A, Pirke KM, Gramsch C, Herz A 1981 β-Endorphin and dynorphin control serum luteinizing hormone level in immature female rats. Nature 294:757–759 [DOI] [PubMed] [Google Scholar]
- Kinoshita F, Nakai Y, Katakami H, Imura H 1982 Suppressive effect of dynorphin-(1-13) on luteinizing hormone release in conscious castrated rats. Life Sci 30:1915–1919 [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A 1982 Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215:413–415 [DOI] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL 2002 Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res 57:235–256 [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM 2009 The role of the brain in female reproductive aging. Mol Cell Endocrinol 299:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G 2010 Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology 58:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E 1975 Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology 96:1073–1087 [DOI] [PubMed] [Google Scholar]
- Knobil E 1980 The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 36:53–88 [DOI] [PubMed] [Google Scholar]
- Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E 1978 The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology 102:52–62 [DOI] [PubMed] [Google Scholar]
- Brenner RM, Carlisle KS, Hess DL, Sandow BA, West NB 1983 Morphology of the oviducts and endometrium of cynomolgus macaques during the menstrual cycle. Biol Reprod 29:1289–1302 [DOI] [PubMed] [Google Scholar]
- Kohama SG, Bethea CL 1995 Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulation of monkey hypothalamus. Endocrinology 136:1790–1800 [DOI] [PubMed] [Google Scholar]
- Noriega NC, Eghlidi DH, Garyfallou VT, Kohama SG, Kryger SG, Urbanski HF 2010 Influence of 17β-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala, and hippocampus of the rhesus macaque. Brain Res 1307:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Noriega NC, Lemos DR, Kohama SG 2009 Gene expression profiling in the rhesus macaque: experimental design considerations. Methods 49:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Urbanski HF 2006 Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol 20:1164–1176 [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF 2006 Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta). Biol Reprod 75:539–546 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E 2009 Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Young 3rd WS 1991 Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- Abel TW, Voytko ML, Rance NE 1999 The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab 84:2111–2118 [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE 2004 Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol 16:146–153 [DOI] [PubMed] [Google Scholar]
- Smith JT, Li Q, Pereira A, Clarke IJ 2009 Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Rance NE 2008 Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 20:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN 2005 Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN 2008 Variations in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E 2002 Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab 87:5160–5167 [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E 2004 Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta). Endocrinology 145:4653–4659 [DOI] [PubMed] [Google Scholar]
- Gill S, Lavoie HB, Bo-Abbas Y, Hall JE 2002 Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab 87:2297–2302 [DOI] [PubMed] [Google Scholar]
- Gill S, Sharpless JL, Rado K, Hall JE 2002 Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 87:2290–2296 [DOI] [PubMed] [Google Scholar]
- Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM, 2009 The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology 150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner JS, Wilson RC, Kaufman JM, Hotchkiss J, Chen Y, Yamamoto H, Pardo RR, Knobil E 1987 Unexpected responses of the hypothalamic gonadotropin-releasing hormone “pulse generator” to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci USA 84:8745–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne KT, Chen MD, Nishihara M, Williams CL, Thalabard JC, Hotchkiss J, Knobil E 1993 Ovarian control of gonadotropin hormone-releasing hormone pulse generator activity in the rhesus monkey: duration of the associated hypothalamic signal. Neuroendocrinology 57:588–592 [DOI] [PubMed] [Google Scholar]