Figure 3.
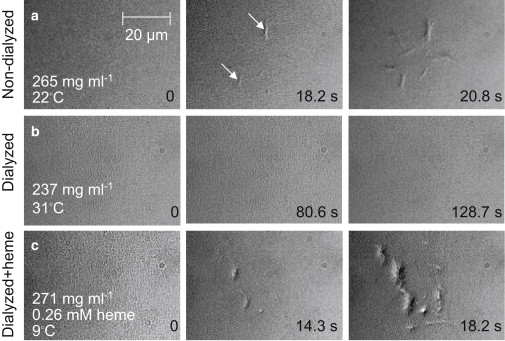
Effects of heme on the polymerization of sickle cell hemoglobin. Solution of CO-HbS is held in slides of uniform 5-μm thickness (32). Photolysis by illumination with a wavelength of 532 nm in the center of the viewfield produces deoxy-HbS, which polymerizes if its concentration is above the solubility point (31,32). Each row represents the evolution of polymerization under a set of conditions. Time after start of photolysis is indicated at each evolution step. HbS concentration and temperature are indicated for each row. White arrows point to some of the HbS polymer domains. (a) HbS polymerization in a solution freshly prepared from stock stored under liquid nitrogen for 1 week. (b) Removal of small molecules by dialysis with a molecular mass cutoff of 2000 g mol−1 prevents polymerization; increasing temperature to 31°C does not lead to polymerization even though, as discussed in the text, higher temperature enhances polymerization; HbS concentration is lower because of dilution during dialysis. (c) After heme at the concentration shown is added to another dialyzed solution sample, polymerization is extremely fast; the temperature was lowered to slow down polymer nucleation and growth so that nucleated polymers would be distinguishable.
