Figure 3.
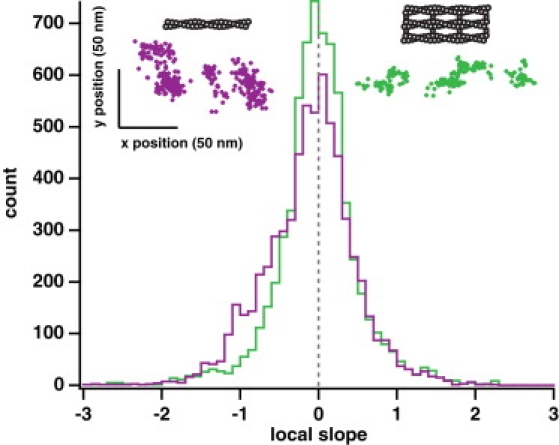
Myosin X takes a spiraling path along single actin filaments. (Upper left, violet), example position trace of an event on a single actin filament. (Upper right, green) Example position trace of an event on a fascin-actin bundle. Each event is moving predominantly in the forward direction (from left to right, as plotted; see Fig. S7 for more examples). The position traces on single actin filaments show local trends of movement forward and to the right (upper left to lower right, as plotted). Local clusters of points within position traces on bundled actin are uncorrelated. Large axes, histograms of the local slope calculated from position traces of events on fascin-actin bundles (green, n = 6675 slopes) and on single actin filaments (violet, n = 6466 slopes). Each slope was calculated using a sliding window of 25 consecutive points in a position trace. Along fascin-actin bundles, the motor shows no asymmetry, with the distribution of slopes centered at 0 (gray dashed line). There is no statistically significant difference between the positive slopes and the absolute value of the negative slopes (p > 0.9 by Wilcoxon rank-sum test). On single actin filaments, the distribution of slopes is asymmetric ∼0. The difference between the positive slopes and the absolute value of the negative slopes is statistically significant (p < 10−34 by Wilcoxon rank-sum test). This shoulder of negative slopes in the distribution demonstrates that the motor is moving to the right locally as it moves forward over short distances, suggesting it turns with the actin helix over the course of a few small steps. The difference between the overall bundle and single filament local slope distributions is statistically significant (p < 10−20 by Wilcoxon rank-sum test). These conclusions do not change when long runs are omitted (>200 points) or the sliding window size is changed (10 or 50 points). The spread in the y position data (∼50 nm) is approximately twice the sum of the actin filament radius (4 nm), the radius of the quantum dot (∼10 nm), and the distance from the binding domain to the C-terminal end of the coiled-coil (∼10 nm or more), and this spread agrees with a recent measurement for the twirling radius of a similarly sized myosin constructs on single actin filaments (41).
