Abstract
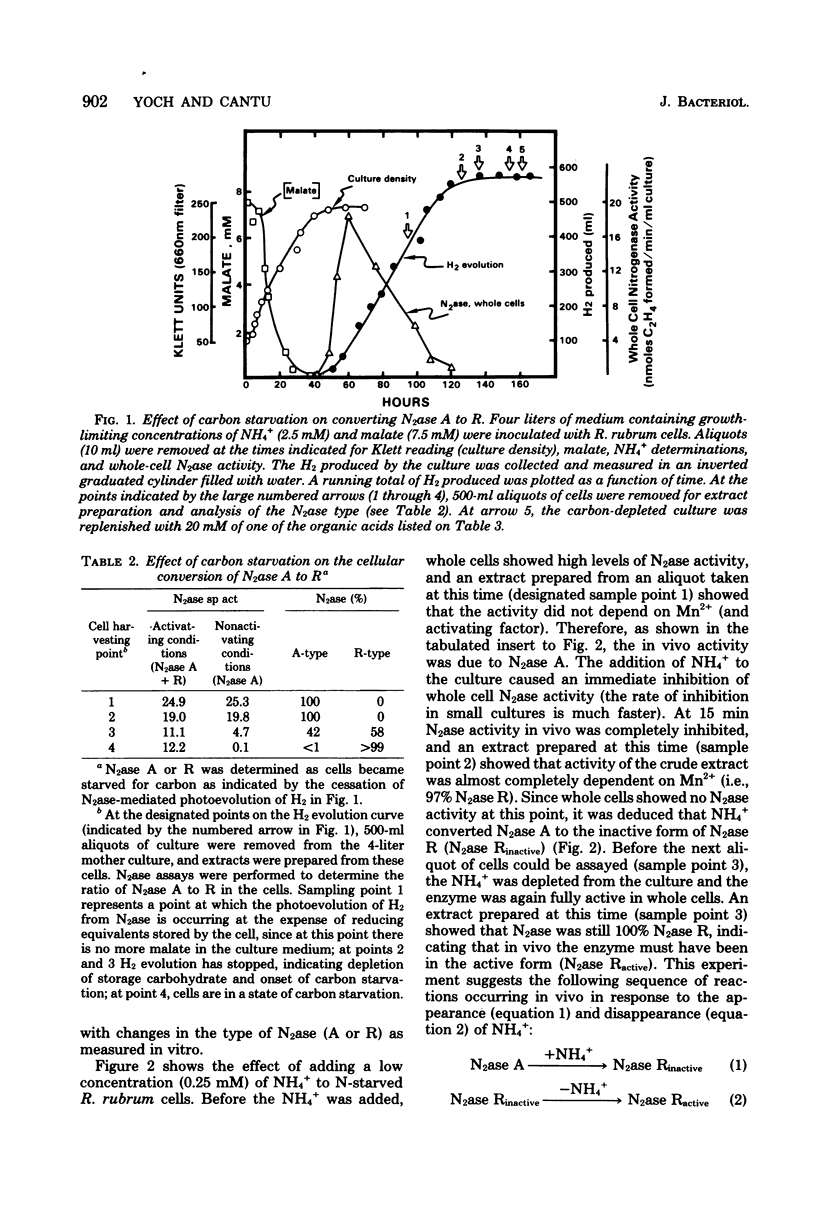
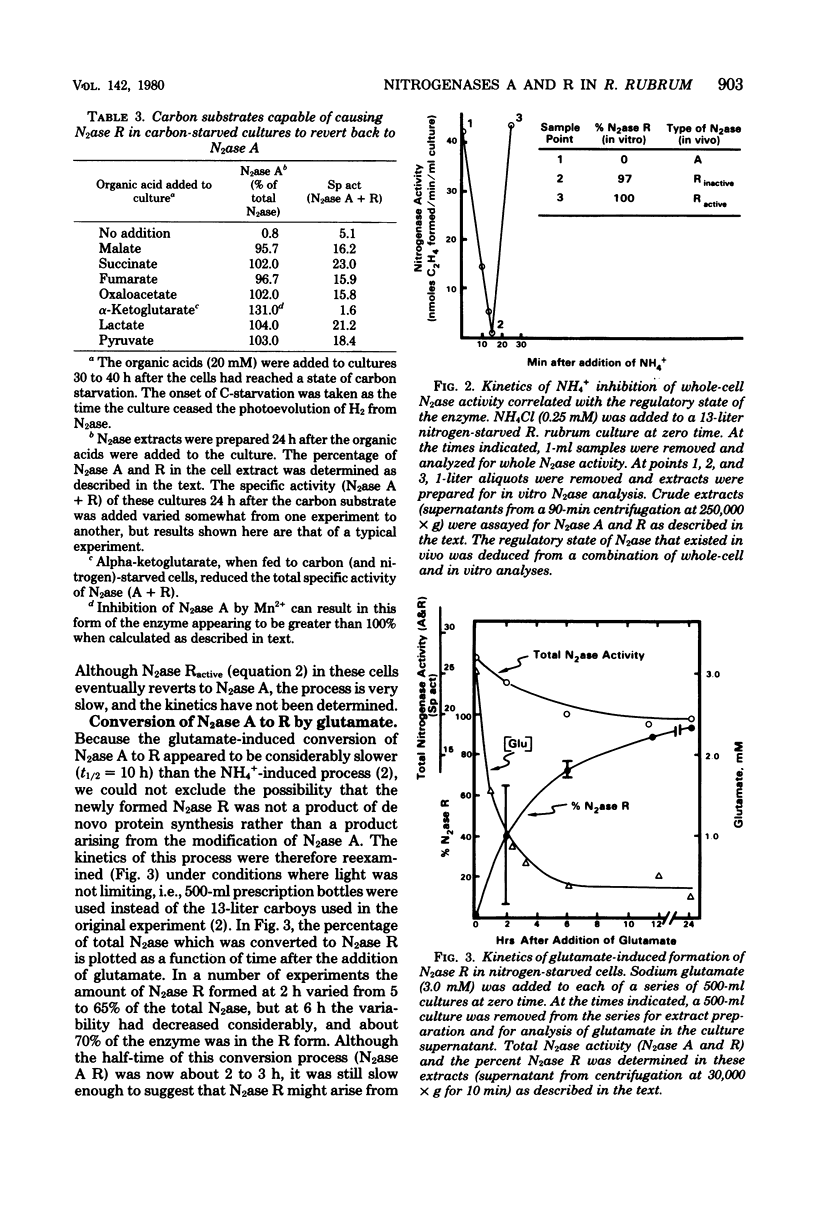
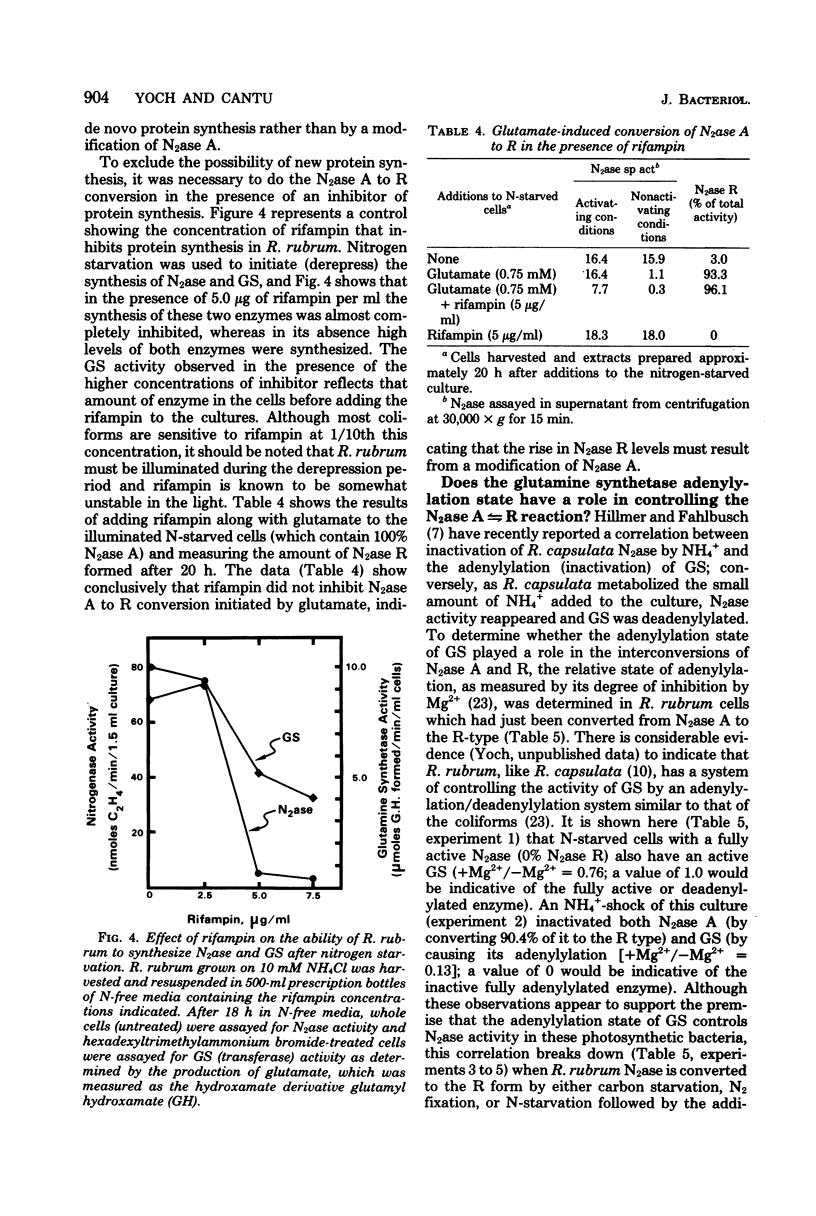
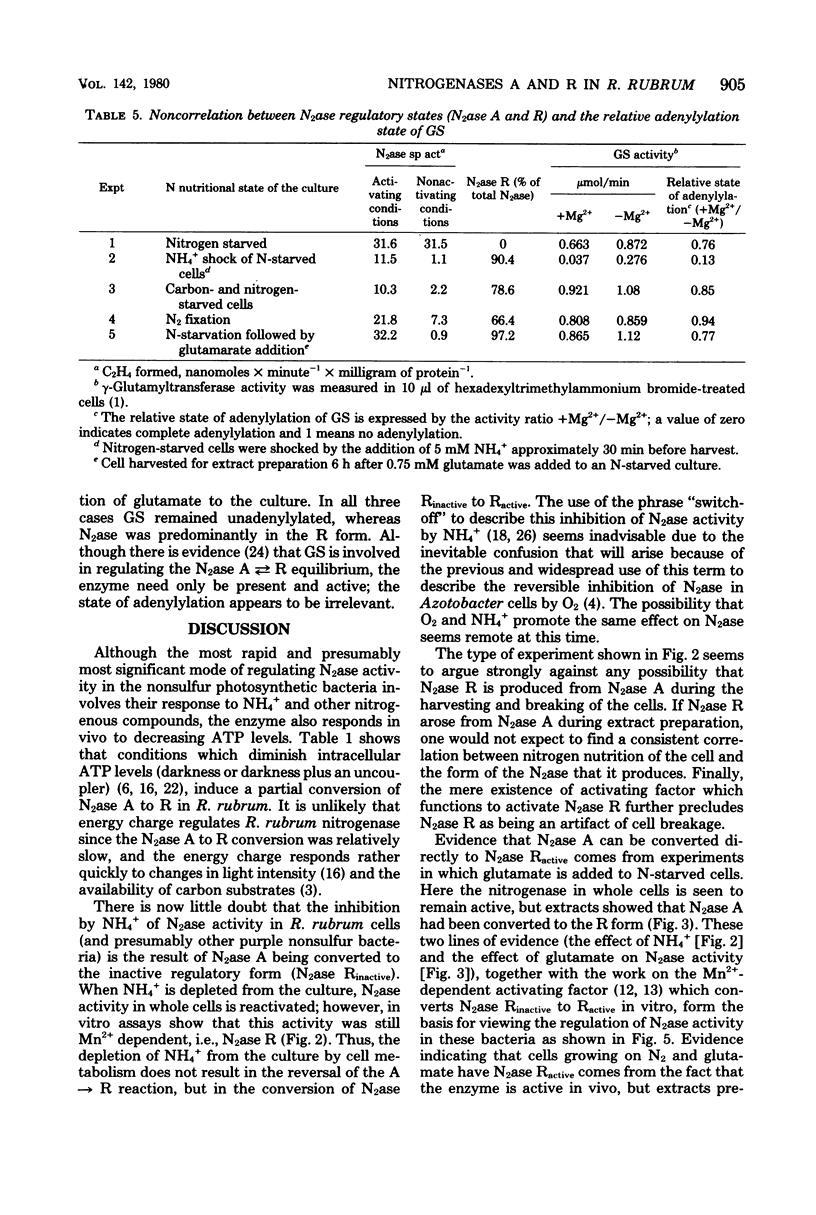
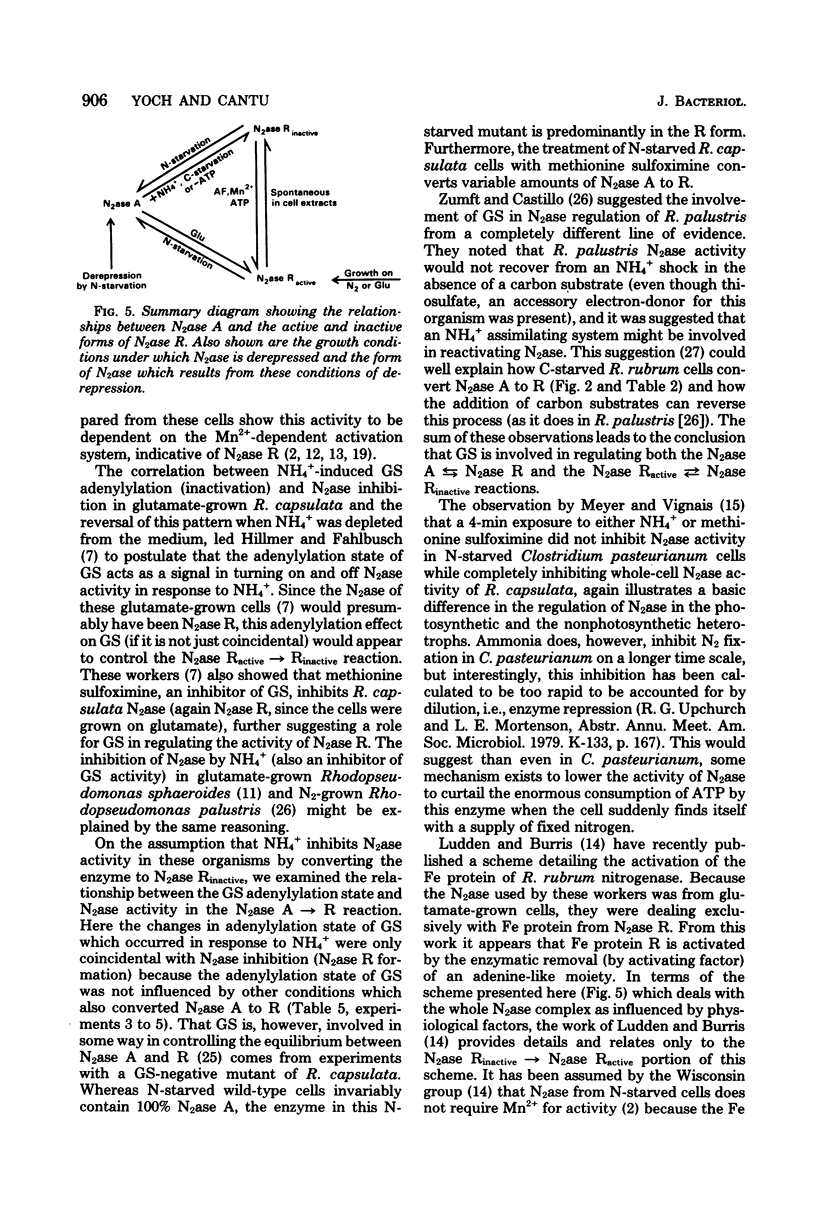
The photosynthetic bacterium Rhodospirillum rubrum regulates the activity of its nitrogenase (N2ase) by interconverting the enzyme into three distinct enzymatic species: N2ase A (a fully active form) and two regulatory forms, N2ase Ractive and N2ase Rinactive. N2ase R is distinguished from N2ase A in vitro by the requirement of its Fe protein for activation by a Mn2+-dependent activating factor. N2ase is converted from the A to the R form in response to certain environmental factors such as carbon starvation, depletion of intracellular adenosine triphosphate, or the addition of NH4+ (or glutamate) to a culture of N-starved cells. The rapid inhibition of R. rubrum N2ase in vivo by NH4+ was shown to result from the conversion of N2ase A to N2ase Rinactive. On depletion of NH4+ from the culture, whole-cell N2ase activity returned; however, the enzyme remained in the R form. Unlike the effect of NH4+, adding glutamate to cells containing N2ase A did not inhibit in vivo activity, but converted the enzyme to the R form (N2ase Ractive). Although glutamate-induced N2ase R formation was much slower than the NH4+-induced reaction, it occurred in the presence of rifampin, indicating that de novo protein synthesis was not involved. This suggested that N2ase R was formed by a modification of N2ase A. Although glutamine synthetase in involved in the conversion of N2ase A to R, the adenylylation state of glutamine synthetase appears not to be involved in regulating this nitrogenase reaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers R. P., Yoch D. C., Arnon D. I. Two forms of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1979 Feb;137(2):779–789. doi: 10.1128/jb.137.2.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd J., Postgate J. R. Interference by oxygen in the acetylene-reduction test for aerobic nitrogen-fixing bacteria. J Gen Microbiol. 1970 Mar;60(3):427–429. doi: 10.1099/00221287-60-3-427. [DOI] [PubMed] [Google Scholar]
- Gibson J., Morita S. Changes in adenine nucleotides of intact Chromatium D produced by illumination. J Bacteriol. 1967 May;93(5):1544–1550. doi: 10.1128/jb.93.5.1544-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer P., Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: production and utilization of H2 by resting cells. J Bacteriol. 1977 Feb;129(2):732–739. doi: 10.1128/jb.129.2.732-739.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Adenylylation/deadenylylation control of the glutamine synthetase of Rhodopseudomonas capsulata. Eur J Biochem. 1977 Dec 1;81(2):365–371. doi: 10.1111/j.1432-1033.1977.tb11960.x. [DOI] [PubMed] [Google Scholar]
- Jones B. L., Monty K. J. Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979 Sep;139(3):1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Removal of an adenine-like molecule during activation of dinitrogenase reductase from Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6201–6205. doi: 10.1073/pnas.76.12.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Vignais P. M. Effects of L-methionine-DL-sulfoximine and beta-N-oxalyl-L-alpha, beta-diaminopropionic acid on nitrogenase biosynthesis and activity in Rhodopseudomonas capsulata. Biochem Biophys Res Commun. 1979 Jul 27;89(2):353–359. doi: 10.1016/0006-291x(79)90637-5. [DOI] [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta. 1977 Oct 12;462(1):187–195. doi: 10.1016/0005-2728(77)90201-8. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U. Nitrogenase from Rhodospirillum rubrum. Relation between 'switch-off' effect and the membrane component. Hydrogen production and acetylene reduction with different nitrogenase component ratios. Biochim Biophys Acta. 1979 Sep 11;547(3):429–437. doi: 10.1016/0005-2728(79)90023-9. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Schön G. Der Einfluss der Kulturbedingungen auf den ATP-, ADP- und AMP-spiegel bei Rhodospirillum rubrum. Arch Mikrobiol. 1969;66(4):348–364. [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Yoch D. C. Manganese, an essential trace element for N2 fixation by Rhodospirillum rubrum and Rhodopseudomonas capsulata: role in nitrogenase regulation. J Bacteriol. 1979 Dec;140(3):987–995. doi: 10.1128/jb.140.3.987-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C. Regulation of nitrogenase A and R concentrations in Rhodopseudomonas capsulata by glutamine synthetase. Biochem J. 1980 Apr 1;187(1):273–276. doi: 10.1042/bj1870273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]


