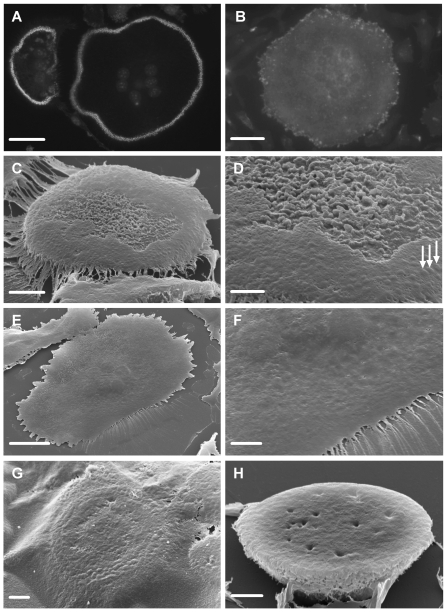
Figure 5. Vitronectin induces formation of ruffled border in osteoclasts.
Glass coverslips were coated with nail-varnish. This was then coated with vitronectin or fibronectin (50 µg/ml). Next, osteoclasts were incubated on this surface for 5 hours in MEM/BSA with M-CSF (50 ng/ml), RANKL (30 ng/ml) and IL-1α (10 ng/ml), with/without the cathepsin inhibitor E64 (3×10−7 M) or calcitonin (100 pg/ml). A,B: Phalloidin-stained preparations after incubation on vitronectin (A) or fibronectin (B). In A, individual podosomes can be discerned within the circumferential belt of podosomes. No podosomes are seen in the osteoclast that had been incubated on fibronectin. C–H: The discs of nail-varnish were separated from the glass coverslip, inverted onto a glass slide, dissolved in acetone, dehydrated in HMDS, and sputter-coated with gold for visualization in the SEM. C,D: undersurface of osteoclast incubated on vitronectin. The central area of the undersurface of the cell is filled with dense membrane folds, while the circumference lacks folds, but shows raised foci likely to represent podosomes (arrows). Note residual film of protein attached to the cell periphery. D: higher magnification of C. Note variation in density of membrane folds in the central area. E,F: Low and higher power view of undersurface of osteoclast incubated on fibronectin. The surface is relatively featureless, and lacks the domain organization apparent after incubation on vitronectin. G: The undersurface of osteoclasts was obscured by a protein film in cultures to which the cathepsin inhibitor E64 was added. Nevertheless, a peripheral belt of raised, podosome-like structures can be discerned through the film. H: The undersurface of osteoclasts incubated with calcitonin lacked membrane ruffles and podosome belts. Scale bars: A,B: 35 µm; C,F,H: 5 µm; D,G: 2 µm; E: 20 µm.