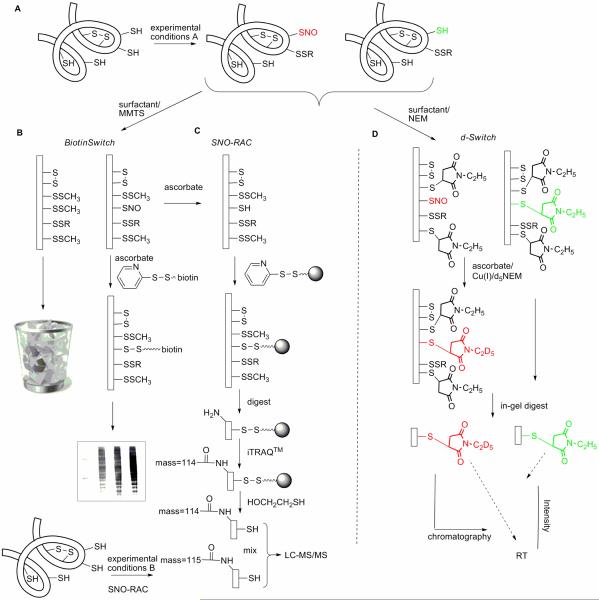
Fig 1. Schematics for biotin switch (BST), SNO-RAC, and d-Switch methods.
A) The proteome may contain cysteines with varied reactivity and accessibility and intramolecular disulfide bonds; BST and modified BST methods discard non-nitrosated proteins. B) The standard BST method visualizes biotinylated proteins by western blot (shown); or may identify proteins by LC-MS/MS chromatography after of pull-down of biotinylated proteins prior to protein digestion. C) The SNO-RAC is a modified BST methodology that uses activated-disulfide beads in place of biotin to isolate nitrosated proteins in combination with iTRAQ™ quantitative proteomic analysis. D) The d-Switch modification is designed to quantify the absolute ratio of nitrosated versus non-nitrosated, reduced cysteine at individual sites in key proteins; therefore, non-nitrosated proteins must be islolated and quantified, not discarded.