Abstract
Current biosynthetic methods for producing proteins containing site-specifically incorporated unnatural amino acids are inefficient because the majority of the amino acid goes unused. Here we present a universal approach to improve the efficiency of such processes using condensed E. coli cultures.
Keywords: unnatural amino acid, tRNA, ubiquitin, aminoacyl-tRNA synthetase
In vivo unnatural amino acid mutagenesis is now an increasingly common technique used to study protein structure and function. Among the many uses are controlling protein function with light1–5, trapping transient protein-protein interactions6, 7, or providing chemically reactive “handles” for the attachment of fluorescent dyes or polymers.8, 9 While some of the amino acids that have been described in the literature are commercially available, many must be prepared by the user through multi-step chemical syntheses. This can complicate or prevent the use of UAAs in laboratories not equipped for synthetic chemistry or at the very least make synthetic UAAs precious materials. Indeed current protocols for in vivo UAA mutagenesis are inefficient in that an extremely small molar equivalent of amino acid is used in the synthesis of protein. Typically millimolar concentrations of UAA are added to protein expression media to produce micromolar or nanomolar yields of protein. In other words, >99% of the synthetic UAA goes unused and is discarded with spent media. Moreover, it is common for protein translation yields using amber codon suppression technology to be significantly lower than that of a wild-type protein.10 Thus any methods that can improve the efficiency of UAA use will be valuable to protein scientists.
In the course of working with synthetic amino acids we were interested if the waste of material used during protein expression could be minimized. Unfortunately the UAA concentration in the media must be at least 1 mM to ensure optimum cell uptake and substrate selection by engineered aminoacyl-tRNA synthtases. The only alternative was to examine if E. coli cultures could be condensed to dramatically smaller volumes than would be typical for protein expression. Such strategies have been used previously to minimize the cost associated with isotopic labeling of protein samples for nuclear magnetic resonance (NMR) studies.11, 12 Provided a robust purification protocol is available, one can generate large quantities of cell mass from small volumes of labeled media.
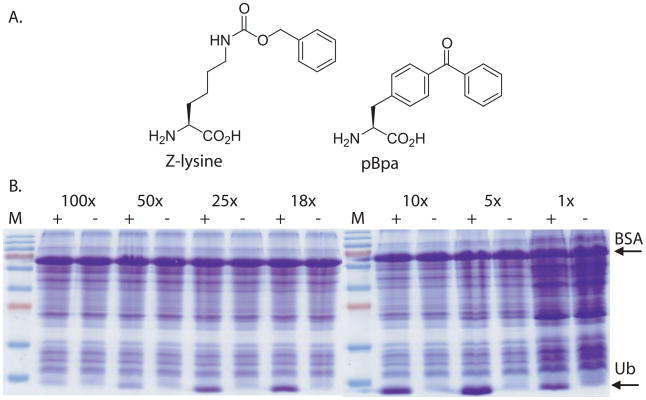
We first chose to conduct protein expression studies using Nε-benzyloxycarbonyl-L-lysine (Z-lysine). This protected lysine analogue has recently been added to the genetic codes of E. coli and mammalian cells using an evolved pyrrolysyl-tRNA synthetase/tRNACUA pair from Methanosarcina species.13, 14 As a protein target we expressed human ubiquitin (Ub) containing a TAG stop codon mutation in place of the codon for Lys 48. Importantly Ub is a small (8 kDa), stable protein that can be easily distinguished from endogenous E. coli proteins in a crude lysate. We performed expressions in a typical rich media format but just prior to induction the cells from an appropriate portion of a larger culture were harvested by centrifugation and then resuspended in a smaller volume (2 mL) of induction media containing 2 mM Z-lysine. For example, to obtain a 50-fold condensation ratio, cells from 100 mL of log-phase culture (OD600 = 0.8) are resuspended in 2 mL resulting in a final OD600 = 40. After expression these cultures were diluted back to the original cell density to allow for equal comparison of all conditions. These samples were then analyzed for protein production using SDS-PAGE of the total cellular protein content. As an internal standard and for quantification purposes we added 10 μg of bovine serum albumin (BSA) to each protein sample.
As can be seen in Figure 1, we surveyed a range of condensation ratios from 1X, or normal cell density (OD600 = 0.8), to ratios of 100X. In the case of Ub, clear protein expression can be seen in all samples except 100X at which point the condensation experiment reaches a point of diminishing returns. This indicates that UAA can be added to cells at the time of induction and be utilized for protein expression at such high cell densities. The lack of protein production in the absence of UAA indicates that the fidelity of amber codon suppression and incorporation is not affected by changes to culture density. We performed these analyses in triplicate and used the presence of a BSA internal standard to quantify protein yields and the relative efficiency of UAA usage under each scenario (Table 1 and Supplemental Information). Using Ub_K48_Z-lysine as an example, we observed that the optimum protein production occurs when cultures are condensed by a factor of 25. These conditions generated 1.8 mg of target protein from a 2 mL expression and represent a significant improvement in yield. Importantly, ~93% less UAA is needed. When expression cultures are not condensed (1X), it is evident from the cell density and the observed protein quantities that there is some continued growth after IPTG induction but little overall increase in protein produced per amino acid.
Figure 1.
A. Structures of Z-lysine and pBpa. B. Protein production of Ub (K48_TAG) in the presence (+) or absence (−) of 2 mM Z-lysine. Total cellular protein observed by Coomassie-stained SDS-PAGE analysis.
Table 1.
| Condensation ratio | Culture loaded (μL) | Protein on gelb (μg) | Total protein producedc (μg) | Total UAA used (μmol) | Protein produced/UAA used (nmol/μmol) |
|---|---|---|---|---|---|
| Ub_K48_Z-Lys | |||||
| 1x | 50 | 3.6 ± 0.7 | 140 | 4 | 4.1 |
| 5x | 20 | 7.4 ± 1.8 | 740 | 4 | 22 |
| 10x | 10 | 6.3 ± 1.7 | 1300 | 4 | 38 |
| 18x | 5.6 | 4.0 ± 0.7 | 1400 | 4 | 41 |
| 25x | 4.0 | 3.6 ± 0.5 | 1800 | 4 | 53 |
| 50x | 2.0 | 1.3 ± 0.3 | 1300 | 4 | 38 |
| 100x | 1.0 | ND | ND | 4 | ND |
| sfGFP_V150_pBpa | |||||
| 1x | 25a | 2.1 ± 0.2 | 170 | 4 | 1.6 |
| 5x | 20 | 3.8 ± 0.5 | 380 | 4 | 3.5 |
| 10x | 10 | 2.5 ± 0.5 | 500 | 4 | 4.6 |
| 18x | 5.6 | 0.7 ± 0.1 | 250 | 4 | 2.3 |
| 25x | 4.0 | ND | ND | 4 | ND |
| 50x | 2.0 | ND | ND | 4 | ND |
| 100x | 1.0 | ND | ND | 4 | ND |
Because this culture grew substantially a smaller sample was loaded. This is considered in the calculations.
Amount of protein on gel was calculated using ImageJ software and normalized to BSA standard (10μg). Average of three experiments ± standard deviation.
Calculated total protein normalized to the full 2 mL expression.
To determine if this methodology is general we next decided to use a different system for UAA mutagenesis coupled with an alternative protein target. We used the Methanococcus jannaschii system developed for genetic encoding of p-benzoylphenylalanine (pBpa)7, to perform mutagenesis on superfolder green fluorescent protein (sfGFP).15 We repeated the condensation experiments using a V150TAG mutant of sfGFP and analyzed triplicate expression experiments by SDS-PAGE (Supplemental Information). We observed a similar trend in the protein yields albeit with lower overall production (Table 1). There was very little protein observed by SDS-PAGE in condensation experiments above 10X. Nevertheless these conditions resulted in a reduced consumption of UAA of ~66%. The lower levels of production observed can be ascribed to an overall lower productivity of this tRNA synthetase/tRNA pair, and/or the relative lower expression of sfGFP in comparison to Ub. In addition, while we do not observe target protein production at the highest condensation ratios, it is possible that there is a low level of production that is not seen in a gel analysis but could be enriched by purification.
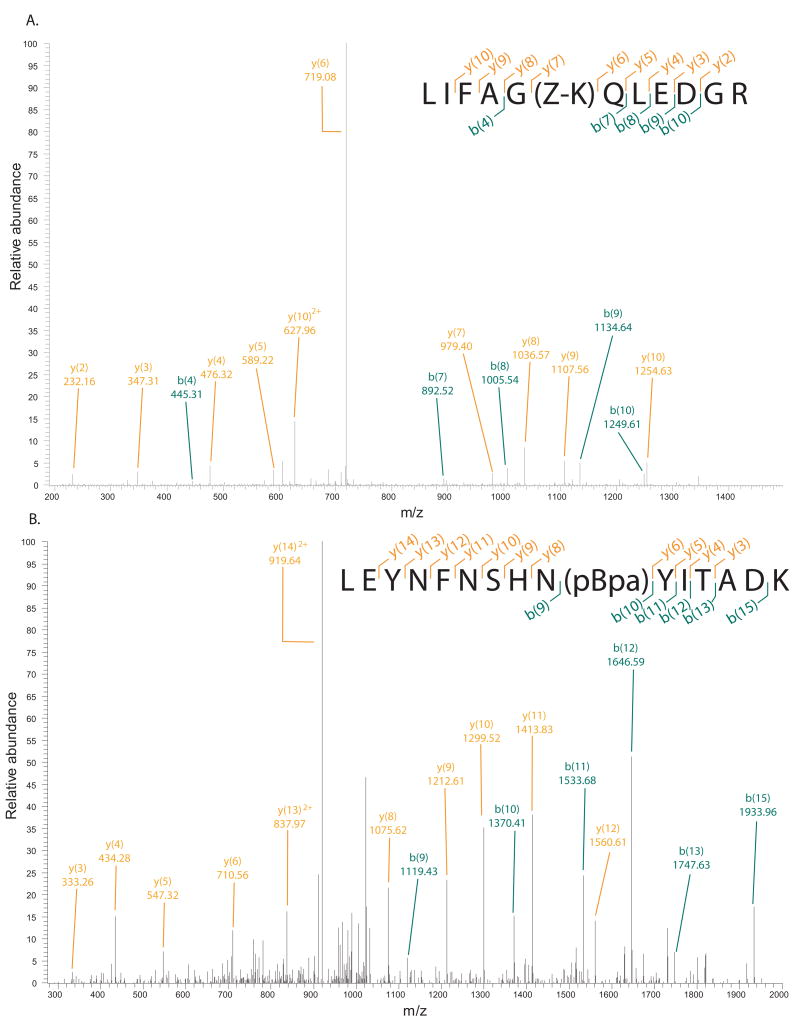
Finally, we decided to conduct “large scale” purifications of both Ub and sfGFP using the optimal condensed formats such that we could verify UAA incorporation by mass spectrometry. The condensed format allowed us to induce 1 L of production cultures of Ub and sfGFP in 40 mL (25X) and 100 mL (10X) of media, respectively. These proteins were purified to homogeneity resulting in large amounts of protein (>25 mgs), a sample of which was subjected to in-gel tryptic digests and the resulting peptides analyzed by mass spectrometry. Upon examining the mass data we observed clear site-specific incorporation of Z-lysine and pBpa into the correct tryptic fragments of Ub and sfGFP, respectively (Figure 2). Importantly we do not see incorporation of any endogenous amino acids at those positions encoded by the amber stop codon, TAG. This again indicates that suppression fidelity remains intact under very high cell density conditions.
Figure 2.
Mass spectral analyses of tryptic fragments of A. Ub_K48_Z-lysine, and B. sfGFP_V150_pBpa.
In summary, the methodology presented here represents a simple yet effective way of maximizing the efficiency of unnatural amino acid mutagenesis studies. This method requires no major changes to experimental conditions and is compatible with standing cell lines and plasmids. The observed improvement using this protocol varies with target protein and UAA used. While it is sufficient to conserve UAA by culture condensation, it might be further enhanced when coupled with codon optimized genes and the Single Protein Production System16 that minimizes expression of endogenous genes during induction. Ideally researchers will test a range of condensation conditions with a protein of interest as described here to optimize production. In addition to protein production, it is possible that condensed E. coli cultures could be used in genetic selections of novel aminoacyl-tRNA synthetase variants. Reporter proteins could be expressed in small volumes and then the cells diluted and selected. In the past such selections have been notorious for consuming large quantities of UAAs. Ultimately we believe that this approach will become standard practice in laboratories that are applying UAAs to solve biophysical problems, particularly in situations where the UAA in use must be chemically synthesized.
Supplementary Material
Acknowledgments
FUNDING INFORMATION: This work was supported by the National Institutes of Health (GM084396 to T.A.C. and GM065334 to D.F.) and the National Science Foundation (CHE-0848398 to T.A.C.).
The authors are grateful to Prof. Peter Schultz (The Scripps Research Institute) for materials. Financial support from the National Institutes of Health (GM084396 and GM065334) and the National Science Foundation (CHE-0848398) is greatly acknowledged.
ABBREVIATIONS
- UAA
unnatural amino acid
- Ub
ubiquitin
- sfGFP
superfolder green fluorescent protein
Footnotes
Experimental procedures for protein expression and calculation of protein yields can be found in the online version, at doi:xxxxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards WF, Young DD, Deiters A. ACS Chem Biol. 2009;4:441. doi: 10.1021/cb900041s. [DOI] [PubMed] [Google Scholar]
- 2.Bose M, Groff D, Xie J, Brustad E, Schultz PG. J Am Chem Soc. 2006;128:388. doi: 10.1021/ja055467u. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins BJ, Marionni S, Young DD, Liu J, Wang Y, Di Salvo ML, Deiters A, Cropp TA. Biochemistry. 2010;49:1557. doi: 10.1021/bi100013s. [DOI] [PubMed] [Google Scholar]
- 4.Chou C, Young DD, Deiters A. Angew Chem Int Ed Engl. 2009;48:5950. doi: 10.1002/anie.200901115. [DOI] [PubMed] [Google Scholar]
- 5.Gautier A, Nguyen DP, Lusic H, An W, Deiters A, Chin JW. J Am Chem Soc. 2010;132:4086. doi: 10.1021/ja910688s. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins BJ, Daggett KA, Cropp TA. Mol Biosyst. 2008;4:934. doi: 10.1039/b801512k. [DOI] [PubMed] [Google Scholar]
- 7.Chin JW, Martin AB, King DS, Wang L, Schultz PG. Proc Natl Acad Sci. 2002;99:11020. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Zhang Z, Brock A, Schultz PG. Proc Natl Acad Sci. 2003;100:56. doi: 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deiters A, Cropp TA, Mukherji M, Chin JW, Anderson JC, Schultz PG. J Am Chem Soc. 2003;125:11782. doi: 10.1021/ja0370037. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Neumann H, Peak-Chew SY, Chin JW. Nat Biotechnol. 2007;25:770. doi: 10.1038/nbt1314. [DOI] [PubMed] [Google Scholar]
- 11.Mao L, Tang Y, Vaiphei ST, Shimazu T, Kim SG, Mani R, Fakhoury E, White E, Montelione GT, Inouye M. J Struct Funct Genomics. 2009;10:281. doi: 10.1007/s10969-009-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M, Roy R, Zheng H, Woychik N, Inouye M. J Biol Chem. 2006;281:37559. doi: 10.1074/jbc.M608806200. [DOI] [PubMed] [Google Scholar]
- 13.Mukai T, Kobayashi T, Hino N, Yanagisawa T, Sakamoto K, Yokoyama S. Biochem Biophys Res Commun. 2008;371:818. doi: 10.1016/j.bbrc.2008.04.164. [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S. Chem Biol. 2008;15:1187. doi: 10.1016/j.chembiol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Nat Biotechnol. 2006;24:79. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Mao L, Inouye M. Nat Protoc. 2007;2:1802. doi: 10.1038/nprot.2007.252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.