Abstract
Nephropathy is a major complication of diabetes mellitus, thus development of rational therapeutic means is critical for improving public health. It was previously reported by us that human mesangial cells locally produced aldosterone, a steroid hormone that plays an important role in the development of diabetic nephropathy. The present experiments clarified the effect of glucose, LDL and angiotensin II, the molecules frequently elevated in patients with diabetic nephropathy, on aldosterone production in human primary mesangial cells. These cells expressed the CYP11B2 mRNA, a rate-limiting enzyme in the aldosterone biosynthesis. LDL and angiotensin II stimulated CYP11B2 mRNA expression in these cells, while a high concentration of glucose, angiotensin II and/or LDL increased aldosterone production. Importantly, atorvastatin (the CAS number: 134523-03–8), an HMG-CoA reductase inhibitor, strongly suppressed their effects on aldosterone production. Atorvastain also suppressed positive effects of these compounds on the mRNA expression of the angiotensin II receptor type 1, thus atorvastatin exerted its negative effect in part through changing expression of this receptor. Since elevated levels of glucose and LDL, and increased action of the renin-angiotensin-aldosterone system is known to participate in the progression of diabetic nephropathy, it is speculated that the mesangial endocrine system that produces aldosterone locally is a promising therapeutic target for diabetic nephropathy where HMG-CoA reductase inhibitors provide an beneficial effect.
Keywords: aldosterone, angiotensin II type 1 receptor, CYP11B2, mesangial cells
1. Introduction
Diabetes mellitus is one of the most popular diseases in western countries, and is frequently accompanied by components of metabolic syndrome, such as central obesity, hypertension and hyperlipidemia [1]. In patients with diabetes mellitus, elevated levels of plasma glucose and subsequent damage of vasculatures causes dysfunction/destruction of several organs, such as the ocular retina, peripheral nerves and the kidney, developing characteristic complications called diabetic retinopathy, neuropathy and nephropathy [1]. Among them, diabetic nephropathy is characterized by reduction in the glomerular filtration rates and development of proteinuria/nephritic syndrome that ultimately leads to complete loss of kidney function and subsequent shift to hemodialysis [2]. Thus, development of rational means for treatment as well as prevention of this complication has strong impact to public health and patients’ quality of life. Patients with diabetic nephropathy frequently develop hypercholesterolemia, thus these patients are sometimes treated with statins, the HMG-CoA reductase inhibitors, which are used for reducing circulating levels of cholesterol [3]. Interestingly, a recent meta-analysis of various clinical trials indicated that this class of chemical compounds reduces proteinuria and preserves renal function in patients with diabetic nephropathy [4]. A clinical trial of GREASE, which investigated the effect of atorvastatin on renal function in diabetic patients with dyslipidemia, also demonstrated that statins prevented progressive reduction of the glomerular filtration rate in these patients [5]. These clinical studies suggest that statins are beneficial for patients with diabetic nephropathy, delaying progression of their renal deterioration. Although exact mechanisms of this effect have not been elucidated as yet, previous reports suggest that statins in part exert their renoprotective effects by directly targeting the kidney independently of their lipid-lowering properties [6–8]. For example, statins in the kidney regulated the expression of the transforming growth factor (TGF)-β that prevented accumulation of macrophages in this organ [8]. Statins also reduced proliferation of mesangial cells and subsequent fibrosis of the glomeruli [7]. Further, these compounds restored physiologic levels of nitric oxide and subsequently prevented renal damage by oxidative stress [8].
Renal glomeruli contain mesangial cells, which play a critical role in the maintenance of renal function, supporting the glomerular capillaries and regulating their blood flow [9]. These cells are recently shown to produce locally aldosterone [10–13], which is one of the steroid hormones classically known to be secreted from the adrenal cortex in response to angiotensin II and the adrenocorticotropic hormone (ACTH) [14]. Systemic aldosterone is one of the causative factors for diabetic nephropathy, by changing local hemodynamics inside the kidney to the direction toward vascular damage and scarring, as well as by injuring the glomerular endothelial cells [15–17]. Aldosterone locally produced from mesangial cells also has a pathologic role in the development of renal diseases, accelerating deposition of the extracellular matrix in the glomeruli in diabetic rats [10, 11]. In addition to mesangial cells, aldosterone is produced in other non-adrenal tissues, such as cardiac myocytes and vascular smooth muscle cells, and underlies development of pathologic conditions in these tissues [18–21]. These pieces of evidence suggest that dysregulation in the production of aldosterone in the kidney, or particularly in mesangial cells, influences development as well as course of various renal diseases, possibly by acting as an auto/paracrine hormone.
In this report, we investigated the effect of atorvastatin (the CAS number: 134523-03–8) on aldosterone production in human primary mesangial cells in the presence of various concentrations of glucose, low-density lipoprotein (LDL) and/or angiotensin II to represent pathologic changes observed in patients with diabetic nephropathy. We found that atorvastatin markedly suppressed aldosterone production stimulated by high glucose, LDL and/or angiotensin II. We suggest that statins may be a promising compound for the treatment of diabetic nephropathy by inhibiting the local aldosterone production stimulated with these agents.
2. Materials and methods
2.1 Materials
All materials and reagents used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise stated. Modified MCDB 131 and the fetal calf serum (FCS) were purchased from Cambrex Co. (East Rutherford, NJ, USA) and San-Ko Pure Chemicals Co. (Tokyo, Japan), respectively. Atorvastatin (the CAS number: 134523-03–8) was kindly provided by Pfizer Inc. (Groton, CT, USA). Human primary mesangial cells (product code: cc-2559) were obtained from Lonza Groups Ltd. (Basel, Switzerland).
2.2 Cell culture
Human primary mesangial cells were cultured in MCDB131 medium containing 5.5 mM glucose, 5% FCS, 50 μg/ml gentamicin sulfate and 50 ng/ml amphotericin-B in a humidified 5% CO2 incubator at 37°C, as previously reported [12]. They were harvested using 0.025% trypsin and 0.01% ethylenediaminetetraacetic acid. Studies were performed using the cells in between 4th and 7th passage.
2.3 Cell number determination
Cell numbers were determined by the CyQUANT cell proliferation assay kit (Molecular Probes, Inc., Eugene, OR, USA).
2.4 Aldosterone production in human primary mesangial cells
Human primary mesangial cells maintained in the regular culture medium were harvested and incubated with Krebs Ringer Buffer-HEPES (KRB-HEPES) in the absence of bovine serum albumin, as previously reported [12]. They were pretreated with indicated concentrations of atorvastatin for 15 min, and were subsequently incubated with various concentrations of LDL, angiotensin II and/or glucose for 4 hrs. Appropriate amounts of mannitol were added for all incubations to the media to keep the same osmolarity as incubating with 25.0 mM glucose. After the incubation, media were collected and aldosterone concentrations were determined by using a specific radioimmunoassay (RIA) (SPAC RIA kit, Daiichi Radio-isotope Co., Tokyo, Japan). Viability of the cells detected by trypan blue exclusion was always greater than 94% throughout the experiments. The lowest aldosterone detection limit of the RIA kit employed was 6.9 fmol/tube, while its overall recovery of aldosterone was 88–94%. Intra-assay and inter-assay variations were 6.5% and 8.2%, respectively.
2.5 Real-time (RT)-PCR for measuring CYP11B2 and AT1R mRNA
Total RNA was purified from human primary mesangial cells and reverse transcription reactions were carried out as previously reported [12]. Quantitative RT-PCRs for determining mRNA levels of the human cytochrome P450, family 11, subfamily B, polypeptide 2 (CYP11B2) and the human angiotensin II receptor type 1 (AT1R) were performed with the FastStart SYBER Green Master reagent (Roche Applied Science, Indianapolis, IN, USA) in the LightCycler 1.5 PCR apparatus (Roche Applied Science) by using specific primer pairs (human CYP11B2: forward primer: 5′-GCTGGATCAGACCCAAGGT-3′, reverse primer: 5′-GGTAGATTTTCTGGATACAGTTGTCA-3′, and human AT1R: forward primer: 5′-CTGGCCCTTTGGCAATTA-3′, reverse primer: 5′-AACACACTAGCGTACAGGTTGAAA-3′). The human GADPH mRNA was employed as an internal control, and was measured by using the following primer pair: forward primer: 5′-AGCCACATCGCTCAGACAC-3′ and reverse primer: 5′-GCCCAATACGACCAAATCC-3′. The obtained Ct (threshold cycle) values of CYP11B2 and AT1R were normalized for those of GAPDH and their relative mRNA expressions were demonstrated as fold induction to the baseline. The dissociation curves of primer pairs used showed a single peak and samples after PCR reactions had a single expected DNA band in an agarose gel analysis (data not shown).
2.6 Statistical analysis
All results are shown as means ± S.D. obtained from at least three different experiments/measurements. Unpaired Student t-test with two tailed values was used for statistical analysis. Differences were considered significant if the p values were less than 0.05.
3. Results
3.1 Human primary mesangial cells express CYP11B2 mRNA
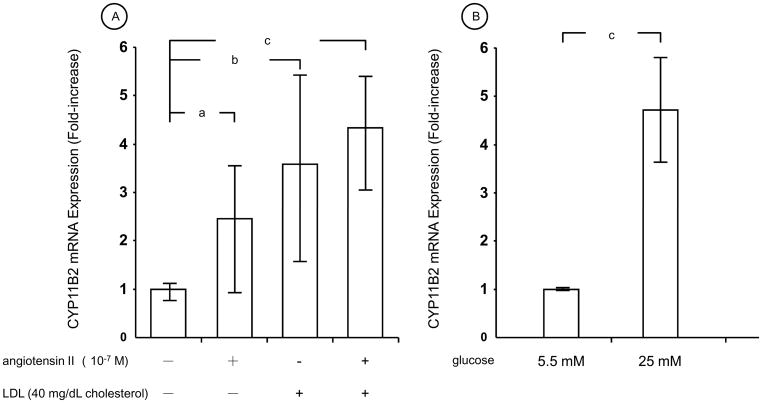
To represent pathologic circumstance of the mesangial cells observed in patients with diabetic nephropathy, we employed culture media containing normal (5.5 mM) or high (25 mM) concentration of glucose (for normal and increased levels of plasma glucose, respectively), and 10 or 40 mg/dL cholesterol of LDL (for elevated levels of serum cholesterol). We also used angiotensin II for stimulating human primary mesangial cells, because this peptide acts a strong stimulator of aldosterone production in these cells [12], and is known to participate in the development of diabetic nephropathy [22, 23]. We first examined the effect of angiotensin II and LDL on the mRNA expression of CYP11B2, the gene encoding a rate-limiting enzyme for the aldosterone biosynthesis [24] (Fig. 1). 10−7 M of angiotensin II or 40 mg cholesterol/dL of LDL increased CYP11B2 mRNA levels in the presence of 5.5 mM glucose (normal glucose), while their simultaneous administration further increased its mRNA expression (Fig. 1A). Twenty-five mM of glucose (high glucose) increased CYP11B2 mRNA expression by 4.7-fold compared to 5.5 mM of glucose (normal glucose) in these cells (Fig. 1B).
Figure 1. Angiotensin II and LDL stimulates mRNA expression of CYP11B2 in human primary mesangial cells.
A: Human primary mesangial cells were incubated for 4 hrs with 10−7 M of angiotensin II and/or 40 mg/dL cholesterol of LDL in the presence of 5.5 mM of glucose with an appropriate amounts of mannitol for adjusting the same osmolarity as 25.0 mM glucose, and mRNA levels of CYP11B2 were determined with RT-PCR. B: Human primary mesangial cells were incubated for 4 hrs with 5.5 mM or 25.0 mM of glucose with an appropriate amounts of mannitol for adjusting the same osmolarity as 25.0 mM glucose, and mRNA levels of CYP11B2 were determined with RT-PCR.
Bars represent mean ± SD values of relative mRNA expression of CYP11B2 shown as fold induction to the baseline obtained in three different experiments. Each experiment was performed as triplicate.
a: p < 0.05, b: p < 0.01, c: p < 0.001, compared to the conditions indicated.
3.2 Incubation with high glucose or angiotensin II increases aldosterone production in human primary mesangial cells, while atorvastatin strongly suppresses it
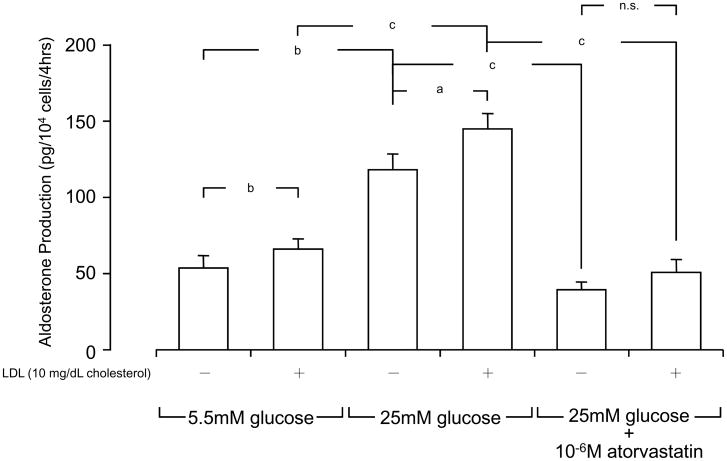
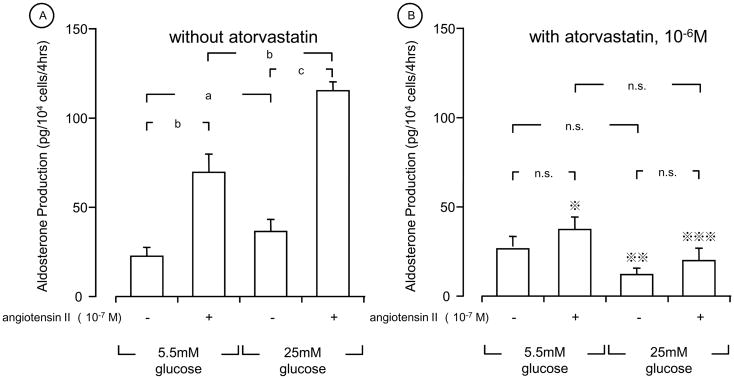
We next examined the effect of glucose and LDL on the aldosterone production in human primary mesangial cells (Fig. 2). These cells produced ~50 pg/104 cells/4hrs of aldosterone in the presence of normal glucose (5.5 mM) and in the absence of 10 mg/dL cholesterol of LDL. Incubation of these cells with high glucose (25 mM) increased aldosterone production by 2.5-fold. Addition of 10 mg cholesterol/dL of LDL increased aldosterone production both at normal and high concentrations of glucose. Atorvastatin strongly suppressed LDL-induced aldosterone production in the presence of high glucose (25 mM) (Fig. 2). Angiotensin II stimulated aldosterone production from human primary mesangial cells in the presence of 10 mg cholesterol/dL of LDL and 5.5 mM of glucose (normal glucose), while it further potentiated the production when the cells were incubated with 25 mM of glucose (high glucose) and the same concentration of LDL (Fig. 3A). Interestingly, pretreatment with 10−6 M of atorvastatin abolished angiotensin II-induced aldosterone production regardless of the concentrations of glucose employed (Fig. 3B).
Figure 2. LDL and high glucose stimulate aldosterone production in human primary mesangial cells, while pretreatment with atorvastatin suppressed their effects.
Human primary mesangial cells were incubated with indicated concentrations of LDL, glucose and/or atorvastatin in the presence of 10 mg/dL cholesterol LDL for 4 hours, and aldosterone production was determined by its specific RIA. All incubations were performed for adjusting the osmolarity by adding mannitol at the same level of 25.0 mM glucose, as described in the legend of Fig. 1. Bars represent mean ± SD values of aldosterone production obtained in three different experiments. Each experiment was performed as triplicate.
a: p < 0.05, b: p < 0.01, c: p < 0.001, n.s: not significant, compared to the conditions indicated.
Figure 3. Angiotensin II and glucose stimulate aldosterone production in human primary mesangial cells, while atorvastatin suppressed their effects.
Human primary mesangial cells were incubated with indicated concentrations of angiotensin II, glucose and/or atorvastatin in the abesnce (A) or presence (B) of 10−6 M of atorvastatin for 4 hours. 10 mg/dL cholesterol of LDL was used throughout the experiment. All incubations were performed for adjusting the osmolarity by adding mannitol at the same level of 25.0 mM glucose, as described in the legend of Fig. 1. Aldosterone production was determined by its specific RIA. Bars represent mean ± SD values of aldosterone production obtained in three different experiments. Each experiment was performed as triplicate.
*: p < 0.05, vs. without atorvastatin **: p < 0.01, vs. without atorvastatin ***: p < 0.001, vs. without atorvastatin
a: p < 0.05, b: p < 0.01, c: p < 0.001, n.s: not significant, compared to the conditions indicated.
3.3 Human primary mesangial cells express AT1R mRNA, while glucose, LDL and atorvastatin modulate it
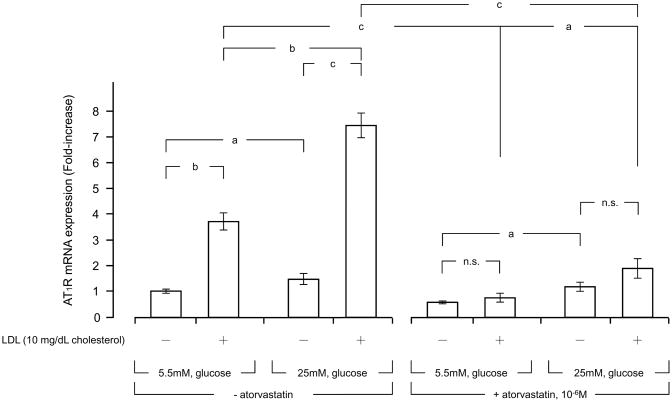
AT1R is the cell surface receptor that mediates the biologic actions of circulating angiotensin II into its responsive organs and tissues [25]. Therefore, we examined mRNA expression of AT1R in human primary mesangial cells. These cells cultured in the presence of 5.5 mM of glucose (normal glucose) expressed AT1R mRNA, while its mRNA expression was significantly elevated (2-fold) when the cells were incubated with 25 mM of glucose (high glucose) (Fig. 4). Addition of 10 mg cholesterol/dL of LDL mildly increased AT1R mRNA expression regardless of the glucose concentrations used. 10−6 M of atorvastatin suppressed AT1R mRNA expression both in the cells incubated with 5.5 mM or 25 mM of glucose, and strongly suppressed LDL-induced potentiation of the mRNA expression in these cells (Fig. 4).
Figure 4. High glucose and LDL stimulate AT1R mRNA expression in human primary mesangial cells, while atorvastatin suppresses their effects.
Human primary mesangial cells were incubated with indicated concentrations of glucose and/or LDL in the presence or absence of 10−6 M of atorvastatin for 4 hours. All incubations were performed for adjusting the osmolarity by adding mannitol at the same level of 25.0 mM glucose, as described in the legend of Fig. 1. mRNA levels of AT1R were determined with RT-PCR. Bars represent mean ± S.D. values of relative mRNA expression of AT1R shown as fold induction to the baseline obtained in three different experiments. Each experiment was performed as triplicate.
a: p < 0.05, b: p < 0.01, c: p < 0.001, n.s: not significant, compared to the conditions indicated.
4. Discussion
We demonstrated that human primary mesangial cells expressed CYP11B2 mRNA and incubation with LDL and/or angiotensin II increased the expression. In agreement with these results, incubation of the cells with high glucose (25 mM) significantly increased aldosterone production compared to their incubation with normal glucose (5.5 mM) both in the absence and presence of 10 mg cholesterol/dl of LDL, whereas angiotensin II significantly enhanced the aldosterone production regardless of the glucose concentrations used. Ten mg/dL cholesterol of LDL had a weak stimulatory effect as well. The HMG-CoA reductase inhibitor atorvastatin strongly suppressed the aldosterone production induced by high glucose or angiotensin II regardless of LDL addition. Thus, human primary mesangial cells produce more aldosterone in the presence of LDL, glucose and/or angiotensin II, and atorvastatin has a diverse suppressive effect on local aldosterone production in the presence or absence of these stimulators. It is likely that the stimulatory effect of LDL and angiotensin II on the aldosterone production may be in some part mediated by their positive influence on CYP11B2 mRNA expression.
Up-regulation of the systemic renin-angiotensin-aldosterone system is one of the most important pathologic changes associated with progression of diabetic nephropathy [15]. Our results suggest that elevated levels of circulating glucose and LDL observed in patients with diabetic nephropathy potentiate angiotensin II-induced secretion of aldosterone from mesangial cells, further worsening their renal disease. The potentiating effect of glucose and LDL may be in part explained by their positive effect on the expression of AT1R mRNA, consistent with previous studies that employed in vivo and in vitro systems [26–28]. The intracellular mechanism(s) underlying the effect of glucose, LDL and angiotensin II on aldosterone production from human primary mesangial cells is (are), however, not known as yet.
We found that atorvastatin suppressed LDL-, high glucose- and/or angiotensin II-induced aldosterone production in human primary mesangial cells. This statin also attenuated their effects on the AT1R mRNA expression in these cells. A previous report indicated that atorvastatin suppressed AT1R mRNA expression in rat aorta and reduced superoxide production in these animals [27]. Further, atorvastatin is also known to prevent angiotensin II-induced vascular remodeling and oxidative stress, and suppressed angiotensin II-mediated activation of the extracellular signal-regulated kinase 1/2 in rat mesenteric arteries [28]. Hypercholesterolemia exacerbates left ventricular remodeling and causes dysfunction of the hearts previously damaged by myocardial infarction through regulation of the AT1R expression, while statins effectively suppressed these hypercholesterolemia-associated changes in the heart [29]. In regard to the kidney, the TNT study demonstrated that 80 mg/day of atorvastatin improved renal function in 10% of patients with chronic kidney diseases, while 10 mg/day of its use improved only in 6.6% [30]. These previous clinical studies suggested that statins are beneficial in the treatment of the cardiac and renal disorders. In agreement with these reports, our in vitro results suggest that statins cause their beneficial effects on the kidney by suppressing local aldosterone production. Elevation of serum glucose levels is a central pathologic change observed in patients with diabetic nephropathy, whereas increased levels of serum cholesterol/LDL frequently accompany to these patients. Thus, these pathologic changes together with angiotensin II, presumably stimulate local glomerular aldosterone production in patients with diabetic nephropathy. As atorvastatin strongly suppressed their effects on local aldosterone and mRNA expression of the AT1R gene, HMG-CoA reductase inhibitors appear be promising therapeutic compounds for the treatment of diabetic nephropathy through suppressing aldosterone production in mesangial cells. On the other hand, we need further experiments, because the present data are too preliminary and demonstrated insufficient data of mechanism(s) clarifying the real mode of action of atorvastatin, which possessed an inhibitory effect on glucose, LDL or angiotensin II-induced aldosterone production in human renal mesangial cells.
In conclusion, we propose that the mesangial endocrine system that produces aldosterone locally is a promising therapeutic target for diabetic nephropathy where HMG-CoA reductase inhibitors provide an beneficial effect.
Acknowledgments
The present study was supported by a Grant-in-Aid for Scientific Research “Adrenal Disorders” provided from the Ministry of Public Health and Labor.
Footnotes
6. Conflict of interest
The authors declare no conflict of interest.
References
- 1.Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan MM, Ballantyne CM. Advances in Lipid Testing and Management in Diabetics. Endocr Pract. 2009:1–38. doi: 10.4158/EP09190.RA. [DOI] [PubMed] [Google Scholar]
- 4.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 5.Athyros VG, Papageorgiou AA, Elisaf M, Mikhailidis DP, Group GSC. Statins and renal function in patients with diabetes mellitus. Curr Med Res Opin. 2003;19:615–617. doi: 10.1185/030079903125002315. [DOI] [PubMed] [Google Scholar]
- 6.Jandeleit-Dahm K, Cao Z, Cox AJ, Kelly DJ, Gilbert RE, Cooper ME. Role of hyperlipidemia in progressive renal disease: focus on diabetic nephropathy. Kidney Int Suppl. 1999;71:S31–36. doi: 10.1046/j.1523-1755.1999.07109.x. [DOI] [PubMed] [Google Scholar]
- 7.Blanco S, Vaquero M, Gomez-Guerrero C, Lopez D, Egido J, Romero R. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens. 2005;18:557–565. doi: 10.1016/j.amjhyper.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Zhou MS, Schuman IH, Jaimes EA, Raij L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-β, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Renal Physiol. 2008;295:F53–59. doi: 10.1152/ajprenal.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlondorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 10.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2008;93:817–824. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu O, Liang X, Dai Y, Liu H, Zang Y, Guo Z, Zhang R, Lai W, Zhang Y, Liu Y. Aldosterone biosynthesis in extra adrenal tissues. Chinese Med J. 1999;112:414–418. [PubMed] [Google Scholar]
- 12.Nishikawa T, Suematsu S, Saito J, Soyama A, Ito H, Kino T, Chrousos G. Human renal mesangial cells produce aldosterone in response to low-density lipoprotein (LDL) J Steroid Biochem Mol Biol. 2005;96:309–316. doi: 10.1016/j.jsbmb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Martinez D, Oestreicher E, Roubsanthisuk W, Yao T, Ricchiuti V, Williams GK. Angiotensin II, aldosterone, and the caveolae, IV. Aldosterone content of the kidney after adrenalectomy. J Hypertens. 2002;20:s115. Abstract#P0457. [Google Scholar]
- 14.Mohaupt MG. The role of adrenal steroidogenesis in arterial hypertension. Endocr Dev. 2008;13:133–144. doi: 10.1159/000134830. [DOI] [PubMed] [Google Scholar]
- 15.Hostetter TH, Rosenberg ME, Ibrahim HN, Juknevicius I. Aldosterone in renal disease. Curr Opin Nephrol Hypertens. 2001;10:105–110. doi: 10.1097/00041552-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hene RJ, Boer P, Koomans HA, Mees EJ. Plasma aldosterone concentrations in chronic renal disease. Kidney Int. 1982;21:98–101. doi: 10.1038/ki.1982.14. [DOI] [PubMed] [Google Scholar]
- 18.Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–2701. doi: 10.1161/01.cir.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 19.Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998;273:4883–4891. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- 20.Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab. 2000;85:2519–2525. doi: 10.1210/jcem.85.7.6663. [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y, Yoneda T, Demura M, Miyamori I, Mabuchi H. Sodium-induced cardiac aldosterone synthesis causes cardiac hypertrophy. Endocrinology. 2000;141:1901–1904. doi: 10.1210/endo.141.5.7529. [DOI] [PubMed] [Google Scholar]
- 22.Bichu P, Nistala R, Khan A, Sowers JR, Whaley-Connell A. Angiotensin receptor blockers for the reduction of proteinuria in diabetic patients with overt nephropathy: results from the AMADEO study. Vasc Health Risk Manag. 2009;5:129–140. [PMC free article] [PubMed] [Google Scholar]
- 23.Shah IM, Mackay SP, McKay GA. Therapeutic strategies in the treatment of diabetic nephropathy - a translational medicine approach. Curr Med Chem. 2009;16:997–1016. doi: 10.2174/092986709787581897. [DOI] [PubMed] [Google Scholar]
- 24.Hakki T, Bernhardt R. CYP17- and CYP11B-dependent steroid hydroxylases as drug development targets. Pharmacol Ther. 2006;111:27–52. doi: 10.1016/j.pharmthera.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 26.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 27.Wassmann S, Laufs U, Baumer AT, Muller K, Konkol C, Sauer H, Bohm M, Nickenig G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59:646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 28.Briones AM, Rodriguez-Criado N, Hernanz R, Garcia-Redondo AB, Rodrigues-Diez RR, Alonso MJ, Egido J, Ruiz-Ortega M, Salaices M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension. 2009;54:142–149. doi: 10.1161/HYPERTENSIONAHA.109.133710. [DOI] [PubMed] [Google Scholar]
- 29.Maczewski M, Maczewska J, Duda M. Hypercholesterolaemia exacerbates ventricular remodelling after myocardial infarction in the rat: role of angiotensin II type 1 receptors. Br J Pharmacol. 2008;154:1640–1648. doi: 10.1038/bjp.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK. Treating to New Targets, I. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol. 2007;2:1131–1139. doi: 10.2215/CJN.04371206. [DOI] [PubMed] [Google Scholar]