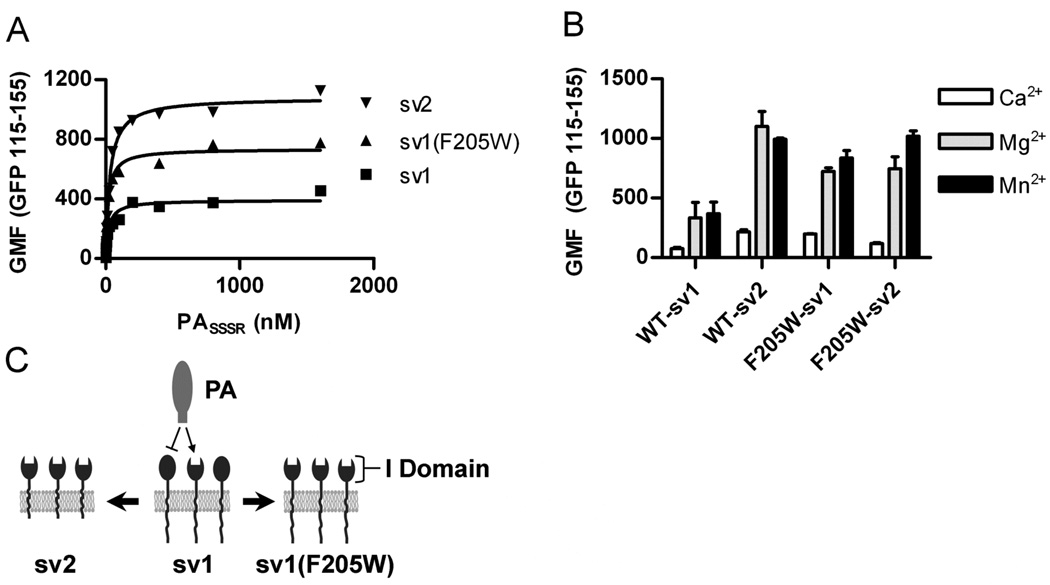
Figure 1. ANTXR1(F205W) is locked into the active conformation.
CHOR1.1 cells expressing the indicated ANTXR1-EGFP fusion proteins were incubated with AlexaFluor 647 labeled PASSSR(K729C) for 6 h on ice, and PA binding was measured by flow cytometry. ANTXR1 expression was normalized by gating on equivalent EGFP signal (115 – 155 relative fluorescence units) from each sample. (A) PA was titrated in the presence of 2 mM MgCl2 and binding measured via flow cytometry. Data shown are representative of two independent experiments with each point corresponding to the geometric mean fluorescence (GMF) of >300 individual cells. (B) The relative contribution of specific cations was determined by incubating 100 nM PA with receptor expressing cells in the presence of 2 mM CaCl2, MgCl2, or MnCl2 and analyzing as in (A). Data points represent the mean ± standard deviation (SD) for three independent experiments. (C) A model depicting the differences in ANTXR1-sv1, -sv2, or the I domain point mutant -sv1(F205W) ability to bind anthrax protective antigen (PA). Two types of receptor activation states exist, relative to PA binding ability. The membrane bound short-tailed sv2 receptors are in the active state and bind PA. The long-tailed sv1 receptors exist in an equilibrium of active and inactive receptors where active receptors are capable of binding PA (arrow) and inactive receptors do not bind PA (line-bar). The I domain point mutation F205W increases the percent of active sv1 receptor capable of binding PA and this overrides inside-out signaling through the cytoplasmic tail, which restricts binding to a proportion of the ANTXR1-sv1. Closed spheres represent inactive receptors whereas open spheres represent active receptors.