Fig. 1.
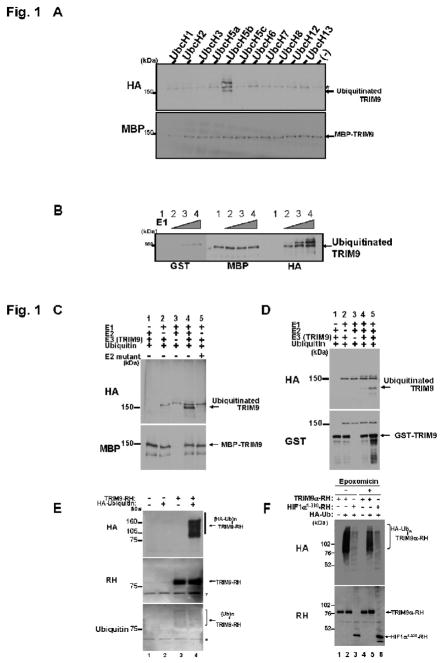
In vitro ubiquitination assay. (A) Determination of E2 enzyme for ubiquitination of TRIM9. MBP-TRIM9 was incubated for 30 min at 37 together with HA-ubiquitin (400 μM), E1 enzyme and the indicated E2 enzymes (100 nM). After incubation, MBP-TRIM9 was solubilized and analyzed by Western blotting using anti-MBP to detect MBP-TRIM9 derivatives and anti-HA to detect ubiquitinated TRIM9. Ubiquitinated TRIM9 is seen only in the presence of UbcH5b as E2 enzyme. An asterisk represents a non-specific band. (B) Ubiquitination of TRIM9 in accordance with increasing amount of E1 enzyme. MBP-TRIM9 was incubated with various amount of E1 enzyme (lane 1, 0 nM; lane 2, 50 nM; lane 3, 100 nM; lane 4, 200 nM). Western blotting using anti-HA antibody shows that ubiquitinated TRIM9 is observed only in the presence of E1 enzyme (lanes 2, 3 and 4). (C) Inability of E2 mutant C85A for ubiquitination of TRIM9. Immunoblot using anti-HA antibody shows that TRIM9 is ubiquitinated in the presence of wild-type UbcH5b (lane 4), but not its mutant C85A (lane 5). (D) Ubiquitination of GST-TRIM9 protein. The complete reaction mixture (lanes 4 and 5) contains HA-ubiquitin, E1 enzyme, UbcH5b as an E2 enzyme, and GST-TRIM9 as a substrate and an E3 enzyme. After incubation, GST-TRIM9 was solubilized and analyzed by Western blotting using anti-GST to detect GST-TRIM9 derivatives and anti-HA to detect ubiquitinated TRIM9. Ubiquitinated TRIM9 is seen only in the presence of E1, E2 and E3 (lanes 4 and 5). Ubiquitination efficiency is enhanced in larger amount of E3 (500 nM in lane 5) than in smaller amount (200 nM in lane 4). (E) E3 activity of TRIM9 in mammalian cells. RH-tagged TRIM9 was expressed with or without HA-tagged ubiquitin in HEK293T cells by plasmid transfection. Twenty-four hours after transfection, the cells were harvested and lysed under denaturing conditions. TRIM9-RH in the lysate was precipitated with cobalt-coated TALON beads and analyzed by immunoblotting using anti-RH antibody to detect both non-ubiquitinated and ubiquitinated TRIM9-RH (middle panel), and anti-HA antibody to detect ubiquitinated TRIM9-RH (top panel). Anti-ubiquitin immunoblot also shows that TRIM9 is ubiquitinated by endogenous ubiquitin (bottom panel, lane 3). Asterisks represent non-specific bands. (HA-Ub)n or (Ub)n indicates poly-ubiquitination. (F) The effect of proteasome inhibitor on the level of TRIM9 protein. Anti-RH immunoblot shows that TRIM9 expression is slightly increased after treatment with 1 μM proteasome inhibitor epoxomicin (lane 5 versus lane 2). HIF1α is used as a positive control for proteasomal degradation. The level of HIF1α is also increased after treatment with a proteasome inhibitor (lane 6 versus lane 3).