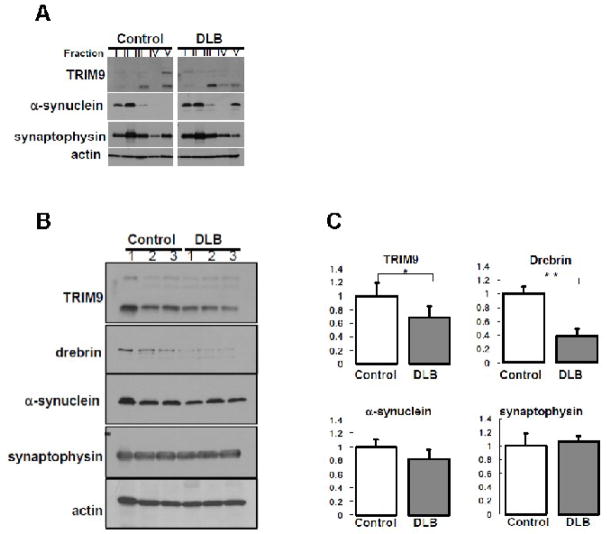
Fig. 5.
(A) Fractionation of human brain samples. Frozen tissues were obtained from the temporal cortex of patients with DLB (n=3) and control subjects (n=3). Sequential biochemical fractionation reveals that TRIM9 protein is composed of soluble and insoluble forms in terms of detergent solubility. The amount of higher band of insoluble TRIM9 and synaptophysin proteins is decreased in a patient with DLB compared with a control subject, whereas insoluble α-synuclein is up-regulated. The amount of lower band of insoluble TRIM9 seems to be decreased in DLB compared with control. Similar results were observed in other cases of DLB and control subjects. Actin is used as control for loading amount. (B) Comparison of expression level of TRIM9 protein in the brain of DLB and controls. Equal amounts of homogenates from human brain were analyzed by immunoblotting using antibodies against TRIM9-C (top), drebrin (second top), α-synuclein (middle), synaptophysin (second bottom), and actin (bottom). The amount of higher and lower bands of TRIM9, drebrin and α-synuclein is decreased in DLB compared with controls. In contrast, the level of synaptophysin is not affected in DLB. Actin is used as control for loading amount. (C) Quantification of the amount of lower band of TRIM9 protein in the brain of DLB and controls. Data are normalized by actin protein level in each sample and indicated by mean + SD. Quantitative analysis shows that the amount of lower band of TRIM9 or drebrin is significantly repressed up to 70% or 40%, respectively, whereas the level of synaptophysin remains stable. * p<0.05, ** p<0.01.