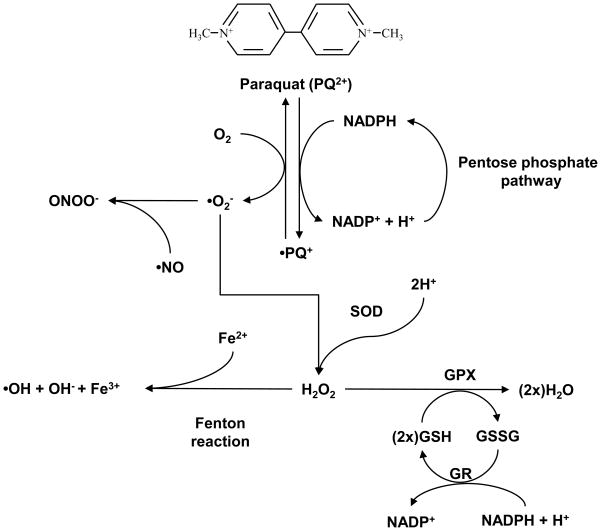
Figure 1. Redox cycling by paraquat.
Paraquat is a highly toxic quarternary nitrogen herbicide which has been shown capable to redox cycle in some cell types. Dication paraquat (PQ2+) is reduced to PQ+ by an NADPH-cytochrome P450 reductase. In the presence of O2, PQ2+ is oxidized with the concomitant production of superoxide anion (•O2−). SOD dismutates •O2− to H2O2 which is further metabolized by GPX. •O2− also reacts •NO leading to the formation of ONOO−. Overexpression of SOD and GPX protects against paraquat-induced toxicity [107]. However, PQ2+ can redox cycle again, leading to a continuous generation of ROS and oxidative stress, and depleting the intracellular pools of GSH and NADPH (required for GSSG recycling to GSH by GR). Accumulation of H2O2 is reduced further to •OH− through Fenton type reactions.