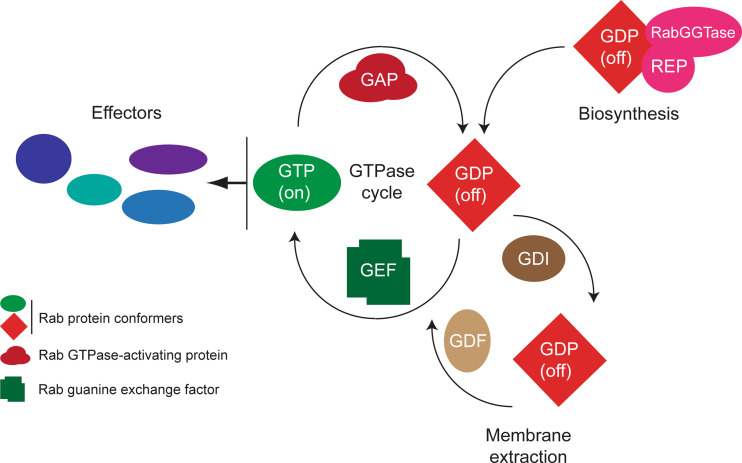
Fig. 4.
Basic functional cycles of Rab proteins. The core Rab function is the GTPase cycle; this process serves to elicit a conformational switch, which importantly regulates interaction with effector molecules (blue lozenges), principally in the GTP-bound (or active ‘on’ state). Switching between these states involves the action of GTPase-activating proteins (GAPs) or guanine nucleotide exchange factors (GEFs), which accelerate the intrinsic hydrolytic activity and nucleotide exchange reactions by several orders of magnitude. A second important cycle is the relocation of the Rab protein following vesicle fusion at the target membrane; usually the Rab is in the GDP, or ‘off’ conformation, which allows specific interactions with a soluble guanine-dissociation inhibitor (GDI) that is able to both sequester the C-terminal prenyl moiety and solubilize the Rab protein. Reintegration into the donor membrane compartment requires connection with a GDI-displacement factor (GDF) and also a GEF, to restore the molecule to the GTP-bound state. Finally, initial targeting following biosynthesis requires insertion utilizing Rab escort protein (REP). Significantly, there are a great number of effector molecules, facilitating a disseminative mode of action for Rab proteins. In most organisms, a similar number of GAPs, GEFs, and Rabs are found in the genome, suggesting an intimate relationship between the GTPase and the factors facilitating the GTPase cycle, while there are usually few GDIs and a single REP