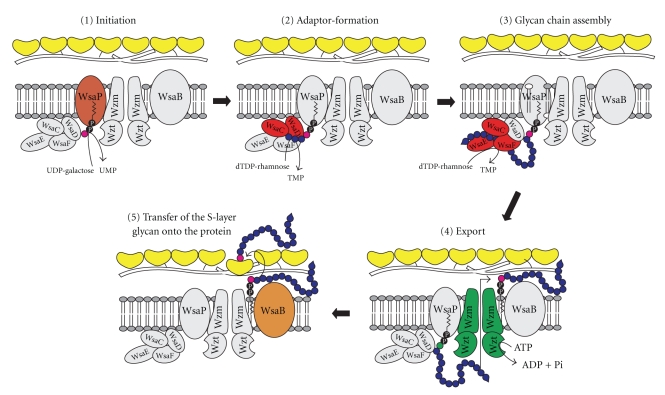
Figure 4.
Proposed model of S-layer glycoprotein glycan biosynthesis in G. stearothermophilus NRS 2004/3a. (1) Initiation: transfer of a Gal residue from UDP-α-D-Gal to a lipid carrier catalyzed by WsaP. (2) Adaptor formation: α1,3-linkage of a rhamnose residue from dTDP-β-L-Rha to the primer by the action of WsaD, followed by the transfer of one or two additional α1,3-linked rhamnoses by the action of WsaC. (3) Glycan chain assembly: formation of repeating unit-like structures by action of the rhamnosyltransferases WsaE and WsaF, whereby WsaE is forming the α1,2- and the α1,3-linkages, and WsaF is forming the α1,2-linkage. Shown is termination of chain growth by 2-O-methylation of the terminal repeating unit, catalyzed by the O-methyltransferase domain of WsaE. (4) Export: the Wzt component of the Wzm/Wzt ABC transporter system is predicted to be responsible for binding of the 2-O-methylated glycan chain and its subsequent export through the membrane. (5) Transfer of the S-layer glycan onto the protein: the final transfer of the completed S-layer glycan to the S-layer protein would be catalyzed by the oligosaccharyltransferase WsaB. This research was originally published in the Journal of Biological Chemistry [35]. The American Society for Biochemistry and Molecular Biology. For colour code see Figure 3; pink, linkage glycose.