Abstract
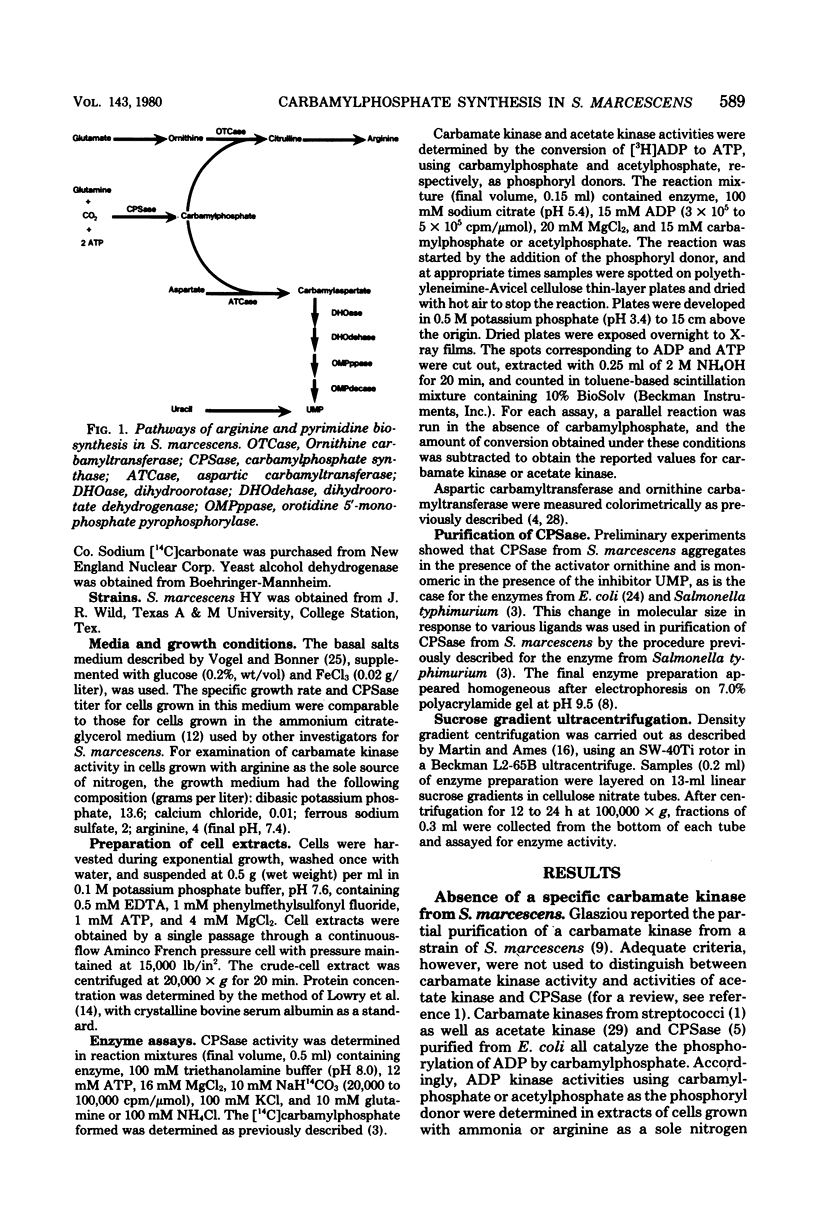
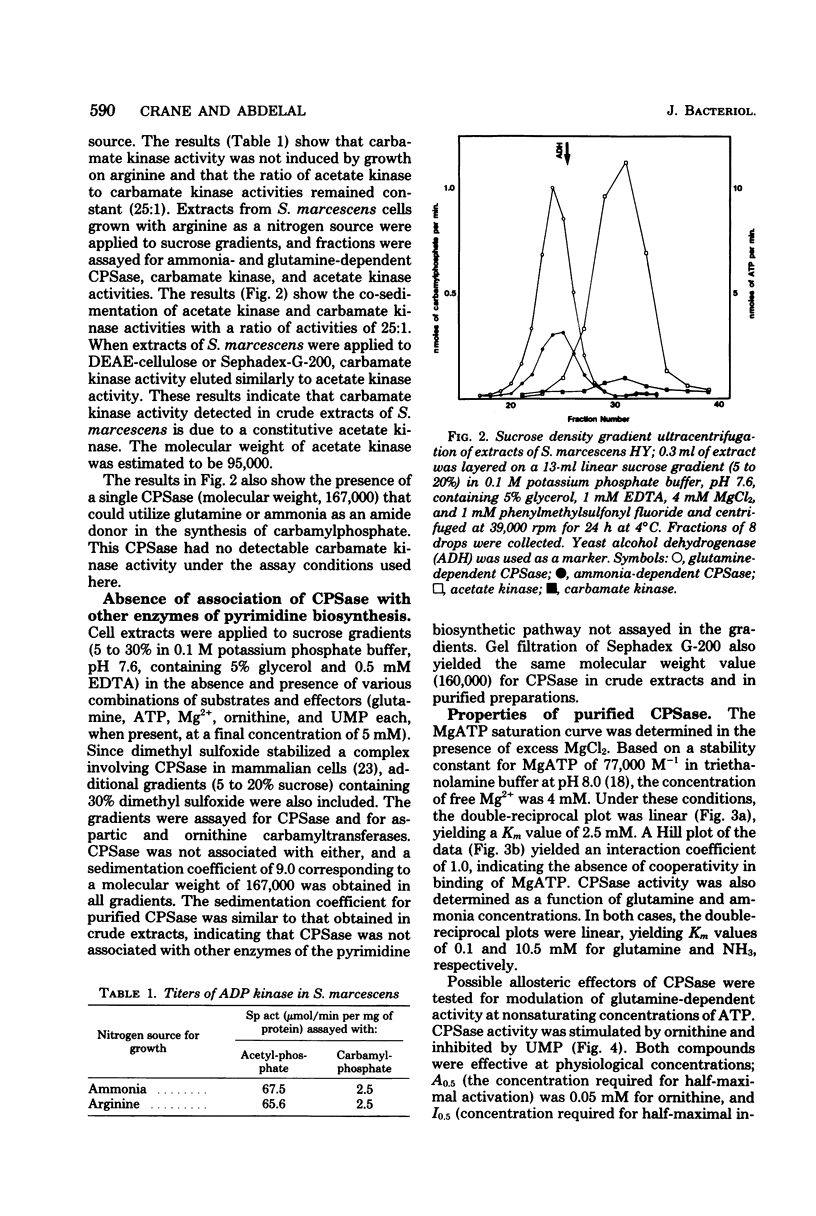
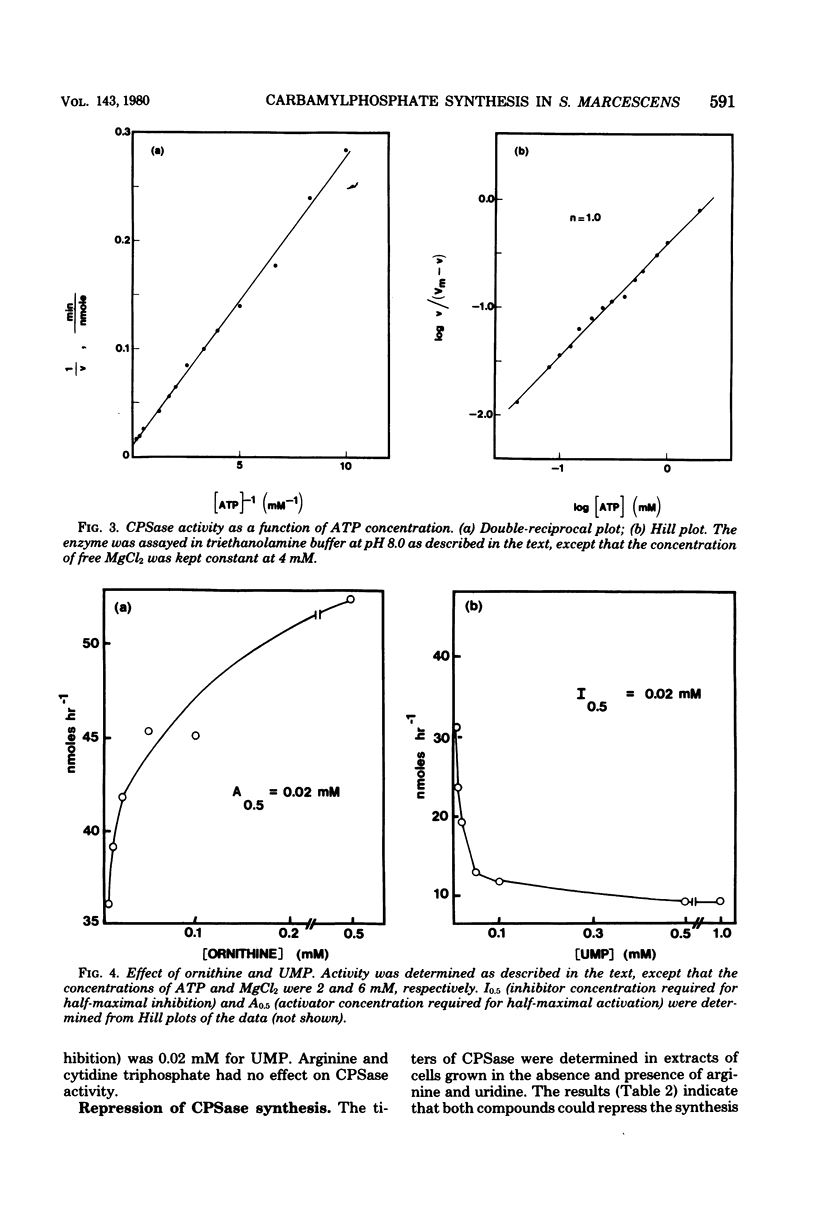
Serratia marcescens HY possessed a single carbamylphosphate synthase (CPSase) which was subject to cumulative repression by arginine and a pyrimidine. CPSase did not appear to be a part of a multifunctional enzyme complex as is the case for other enzymes of pyrimidine biosynthesis in this organism. CPSase was purified to homogeneity. The molecular weight of the enzyme was estimated to be 167,000 by sucrose density gradient ultracentrifugation. The double-reciprocal plot for magnesium adenosine triphosphate was linear, yielding a Km value of 2.5 mM. The enzyme utilized either glutamine (Km, 0.1 mM) or NH3 (Km, 10.5 mM) as a nitrogen donor in the reaction. CPSase activity was subject to activation by ornithine and feedback inhibition by uridine monophosphate, as is the case for other enteric bacteria. Carbamate kinase activity, detected in crude extracts of S. marcescens, was shown to be due to a constitutive acetate kinase. The absence of carbamate kinase from S. marcescens HY is consistent with the inability of this organism to utilize arginine as a source of energy under anaerobic conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Abdelal A. T., Kennedy E. H., Nainan O. Ornithine transcarbamylase from Salmonella typhimurium: purification, subunit composition, kinetic analysis, and immunological cross-reactivity. J Bacteriol. 1977 Mar;129(3):1387–1396. doi: 10.1128/jb.129.3.1387-1396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Bicarbonate-dependent cleavage of adenosine triphosphate and other reactions catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1966 Oct;5(10):3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- KALMAN S. M., DUFFIELD P. H., BRZOZOWSKI T. IDENTITY IN ESCHERICHIA COLI OF CARBAMYL PHOSPHOKINASE AND AN ACTIVITY WHICH CATALYZES AMINO GROUP TRANSFER FROM GLUTAMINE TO ORNITHINE IN CITRULLINE SYNTHESIS. Biochem Biophys Res Commun. 1965 Feb 17;18:530–537. doi: 10.1016/0006-291x(65)90786-2. [DOI] [PubMed] [Google Scholar]
- LABRUM E. L., BUNTING M. I. Spontaneous and induced color-variation of the HY strain of Serratia marcescens. J Bacteriol. 1953 Apr;65(4):394–404. doi: 10.1128/jb.65.4.394-404.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Characterization of pyrimidine-repressible and arginine-repressible carbamyl phosphate synthetases from Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):82–91. doi: 10.1128/jb.137.1.82-91.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Schröter B. Structure-function relationships in the arginine pathway carbamoylphosphate synthase of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):167–176. doi: 10.1128/jb.134.1.167-176.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Holwell J. H., Abdelal A. T. Purification and properties of the arginine-specific carbamoyl-phosphate synthase from Saccharomyces cerevisiae. J Gen Microbiol. 1978 May;106(1):145–151. doi: 10.1099/00221287-106-1-145. [DOI] [PubMed] [Google Scholar]
- Shoaf W. T., Jones M. E. Uridylic acid synthesis in Ehrlich ascites carcinoma. Properties, subcellular distribution, and nature of enzyme complexes of the six biosynthetic enzymes. Biochemistry. 1973 Oct 9;12(21):4039–4051. doi: 10.1021/bi00745a004. [DOI] [PubMed] [Google Scholar]
- THORNE K. J., JONES M. E. CARBAMYL AND ACETYL PHOSPHOKINASE ACTIVITIES OF STREPTOCOCCUS FAECALIS AND ESCHERICHIA COLI. J Biol Chem. 1963 Sep;238:2992–2998. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wild J. R., Belser W. L., O'Donovan G. A. Unique aspects of the regulation of the aspartate transcarbamylase of Serratia marcescens. J Bacteriol. 1976 Dec;128(3):766–775. doi: 10.1128/jb.128.3.766-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J. R., Belser W. L. Pyrimidine biosynthesis in Serratia marcescens: a possible role for nonsequential enzyme interactions in mimicking coordinate gene expression. Biochem Genet. 1977 Feb;15(1-2):157–172. doi: 10.1007/BF00484559. [DOI] [PubMed] [Google Scholar]
- Wild J. R., Belser W. L. Pyrimidine biosynthesis in Serratia marcescens: polypeptide interactions of three nonsequential enzymes. Biochem Genet. 1977 Feb;15(1-2):173–193. doi: 10.1007/BF00484560. [DOI] [PubMed] [Google Scholar]
- YASHPHE J., GORINI L. PHOSPHORYLATION OF CARBAMATE IN VIVO AND IN VITRO. J Biol Chem. 1965 Apr;240:1681–1686. [PubMed] [Google Scholar]


