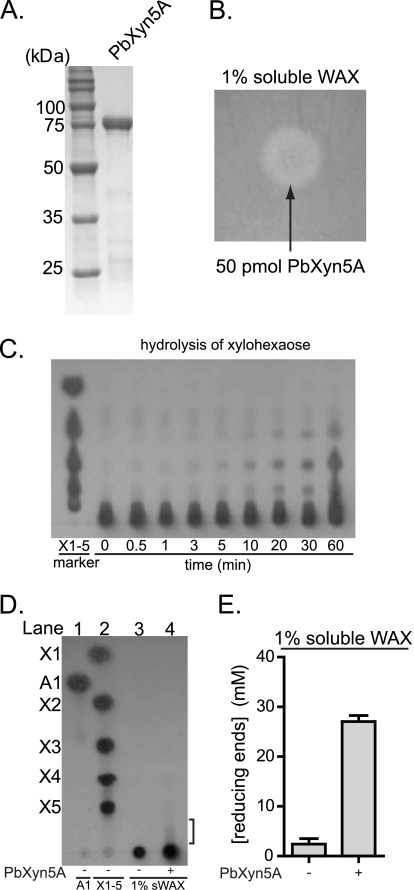
FIGURE 3.
P. bryantii B14 ORF0150 encodes an enzyme with endoxylanase activity. A, purification of recombinant PbXyn5A. ORF0150 was cloned into an expression vector and expressed heterologously as a hexahistidine fusion protein in E. coli. The protein (PbXyn5A) was purified using cobalt affinity chromatography, and the eluate was analyzed by 12% SDS-PAGE, followed by Coomassie Brilliant Blue G-250 staining. B, depolymerization of soluble wheat arabinoxylan. PbXyn5A was assessed for its capacity to depolymerize soluble WAX by incubating the protein on an agar plate infused with WAX followed by staining and destaining with Congo red and 1 m NaCl, respectively. C, hydrolysis of xylohexaose. PbXyn5A-catalyzed hydrolysis of xylohexaose was assessed by incubating the enzyme with the substrate, removing aliquots at the indicated time points, and then resolving the products by thin layer chromatography followed by staining with methanolic orcinol. D, thin layer chromatography of products released from WAX by PbXyn5A. PbXyn5A (0.50 μm) was incubated with WAX (1% w/v), and the products were resolved by thin layer chromatography followed by staining with methanolic orcinol. Xylo-oligosaccharide standards X1–X5 and arabinose (A1) were spotted on the plate in lanes 2 and 1, respectively, to serve as markers for the identification of hydrolysis products. In lane 4, PbXyn5A was incubated with WAX at 37 °C for 15 h, and 2.5 μl of the reaction mixture were resolved on the TLC plate. E, reducing sugars released from WAX by PbXyn5A. Wild-type Xyn5A was incubated with WAX (1% w/v), and the amounts of reducing sugars released were determined by the para-hydroxybenzoic acid hydrazide assay. The reducing sugar concentrations were calculated from the absorbance at 410 nm by comparison to a standard curve generated with known concentrations of glucose. E, the values are reported as the means ± S.D. from three independent experiments.