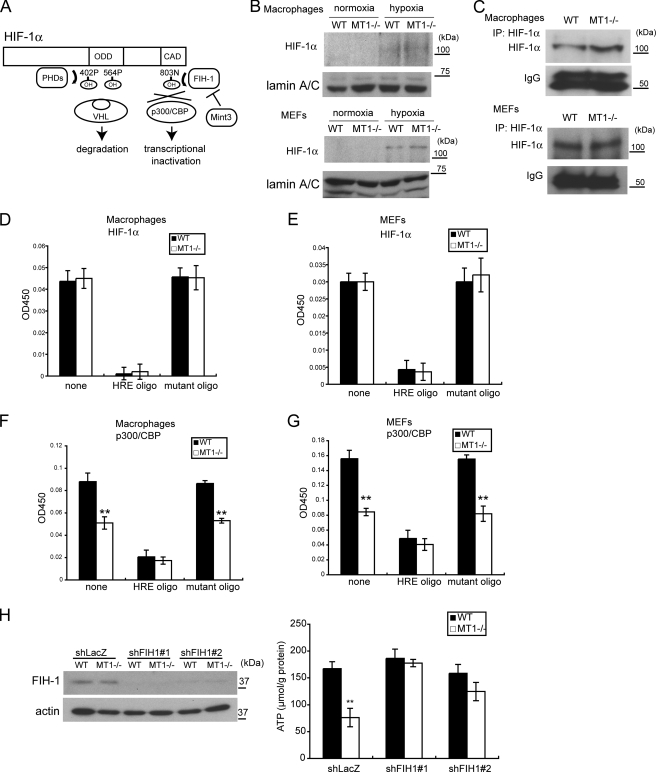
FIGURE 2.
MT1-MMP regulates ATP production through an effect upon HIF-1α. A, schematic depiction of how HIF-1α is regulated by PHDs and FIH-1. ODD, oxygen-dependent degradation domain. B, the amount of nuclear HIF-1α protein is low during normoxia in WT and MT1−/− macrophages (top) and MEFs (bottom). Nuclear extracts were subjected to Western blot for detection of HIF-1α and lamin A/C. Hypoxia increased the amount of HIF-1α but not lamin A/C. C, detection of nuclear HIF-1α protein in cells cultured during normoxia. 40-fold more nuclear extract than that used in the former experiment (B) was subjected to immunoprecipitation (IP: HIF-1α) to concentrate the protein, followed by Western blot for detection. D and E, the presence or absence of MT1-MMP does not affect the amount of HIF-1α that can bind HRE. Nuclear extracts from WT and MT1−/− macrophages (D) and MEFs (E) were subjected to the plate assay. Free HRE or mutant oligonucleotides were used as competitors. F and G, the amount of p300/CBP associated with HIF-1α is affected by MT1-MMP. Nuclear extracts from WT and MT1−/− macrophages (F) and MEFs (G) were subjected to the plate assay. H, FIH-1 activity is reduced in WT macrophages compared with that observed in MT1−/− cells. FIH-1 protein was detected by Western blot as in the left panel. Expression of FIH-1 was knocked down using two shRNA sequences (#1 and #2). Down-regulation of FIH-1 results in greater ATP production (right). Note that the level of ATP in WT cells is comparable with that observed in the FIH-1 knockdown cells. shRNA targeted to the LacZ mRNA (shLacZ) served as a negative control. The data in D–H were analyzed using Student's t test. *, p < 0.05; **, p < 0.01.