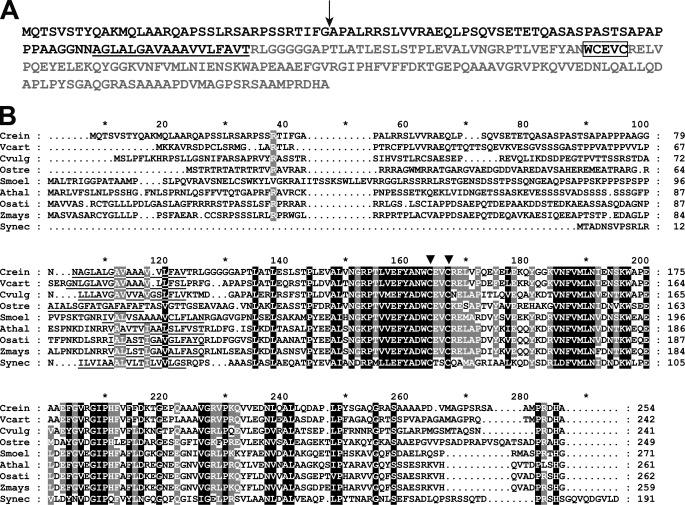
FIGURE 4.
CCS5 is a thioredoxin-like protein conserved in the green lineage. A, amino acid sequence of Chlamydomonas CCS5. The amino acid sequence of Chlamydomonas CCS5 protein was deduced from assembly of a full-length cDNA. The cleavage site of the plastid targeting sequence predicted by PredSL (65) is indicated by a black arrow, and the putative transmembrane domain is underlined. The hydrophilic C-terminal domain predicted to be facing the lumenal side of the thylakoid membrane is highlighted in light gray. The thioredoxin motif with active cysteines is boxed. B, alignment of CCS5/HCF164-like proteins. Sequences of CCS5/HCF164 from C. reinhardtii (Crein), Volvox carteri (Vcart, 80757), Chlorella vulgaris (Cvulg, 79381), Ostreococcus RCC809 (Ostre, 68525), Selaginella moellendorffii (Smoel, 159329), A. thaliana (Athal, CAC19858), Oriza sativa (Osati, NP_001051387), Zea mays (Zmays, NP_001152226), and TxlA from Synechococcus sp. PCC 7942 (Synec, U05044) were aligned using the ClustalW algorithm (Blosum62 scoring matrix) in Bioedit. The alignment was edited using the GeneDoc multiple alignment editor. Amino acids strictly conserved in all sequences are shaded in black and those conserved in the majority of the sequences (7 of 9) are shaded gray. Asterisk marks residues 10, 30, 50, 70, etc. The putative membrane anchor is underlined. Two downward arrowheads indicate the cysteines in the WCXXC motif.