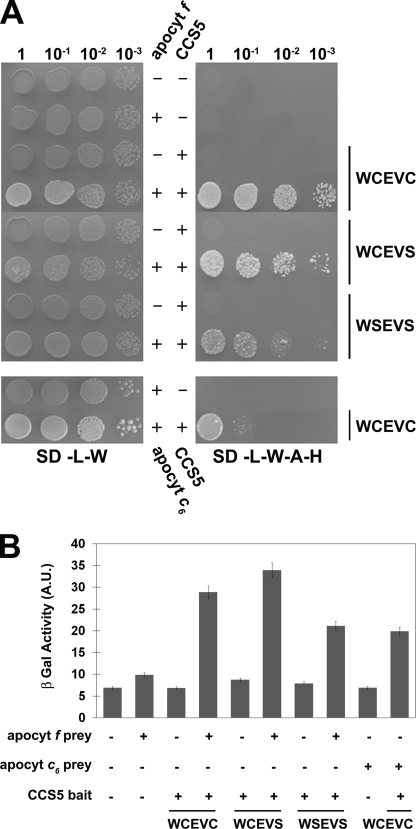
FIGURE 7.
CCS5 interacts with apocytochrome f and c6 in a yeast two-hybrid assay. The soluble domain of Chlamydomonas wild type (WCEVC) or cysteine-less CCS5 (WCEVS and WSEVS) constitutes the bait and is expressed as a fusion with the GAL4 DNA binding domain from the TRP1-based pGBKT7 vector. The soluble domain of Chlamydomonas apocytochrome (apocyt) f or c6 constitutes the prey and is expressed as a fusion with the GAL4 activation domain from the LEU2-based pGADT7 vector. The yeast PJ69-4A reporter strain was co-transformed with various combinations of two-hybrid plasmids. + refers to the presence of the bait or prey expressing constructs, and − refers to the presence of pGBKT7 or pGADT7 vectors in the yeast transformants. A, GAL4-dependent adenine and histidine prototrophies indicate interaction between apocytochrome f/c6 and CCS5. The yeast transformants were tested for adenine/histidine prototrophies that depend upon reconstitution of an active GAL4. 10-Fold dilution series of one representative transformant for each combination were plated on solid medium lacking leucine and tryptophan (SD-L-W) or lacking leucine, tryptophan, adenine, and histidine (SD-L-W-A-H) and incubated at 28 °C for 2 or 4 days, respectively. B, GAL4-dependent β-galactosidase activities indicate interaction between apocytochrome f/c6 and CCS5. The yeast PJ69-4A transformants were tested for β-galactosidase activity that depends upon reconstitution of an active GAL4. 2-Nitrophenyl β-d-galactopyranoside was used as a substrate. β-Galactosidase (β-Gal) activity is expressed in arbitrary units (A.U.). The values displayed are the average β-galactosidase activities from three individual transformants with standard deviation indicated by error bars.