Figure 6. Synergy Between Anti-CD47 Antibody and Rituximab Does Not Occur Through NK Cells or Complement.
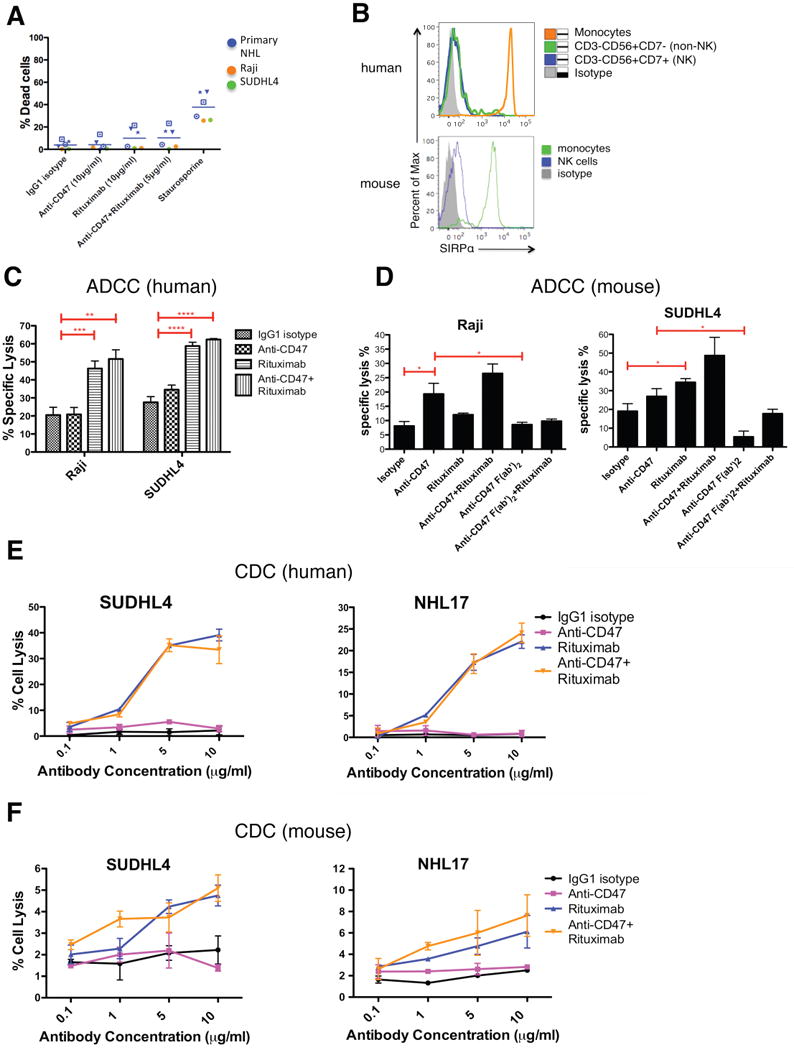
(A) NHL cells were incubated with the indicated soluble antibodies for 2 hours and the percentage of dead cells was calculated (% Annexin V+ and/or 7-AAD+). No statistically significant difference in % dead cells was observed with the combination of anti-CD47 antibody and rituximab compared to either anti-CD47 antibody alone (p=0.24) or rituximab alone (p=0.95). (B) SIRPα expression is shown for both human and mouse NK cells as determined by flow cytometry. (C,D) Chromium release assays measuring ADCC were performed in triplicate with human (C) and mouse (D) at an effector:target ratio of 17.5:1 and percent specific lysis is reported. Antibodies were incubated at 10μg/ml except anti-CD47 full length or F(ab′)2 antibody+rituximab (5μg/ml). (E) CDC assay with human complement was performed in duplicate. Compared to IgG1 isotype control, anti-CD47 antibody did not enable CDC (p>0.2), while rituximab did (p<0.001) by 2-way ANOVA for both SUDHL4 and NHL17*. Combination treatment with anti-CD47 antibody and rituximab did not enable greater levels of CDC compared to rituximab (p=0.78). (F) CDC assay with mouse complement was performed in duplicate. Compared to IgG1 isotype control, anti-CD47 antibody did not enable CDC (p>0.25) while rituximab did (p=0.03, p=0.08, respectively) for both SUDHL4 and NHL17*. No difference in CDC between CD47 antibody+rituximab and rituximab alone was observed (p>0.13) for both SUDHL4 and NHL17*. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. NHL17*=Primary NHL17 cells expanded in culture. See also Figure S6.
