Abstract
Background and aim: The diagnosis of peripheral diabetic neuropathy is based on clinical examination. Nerve conduction study (NCS) enables earlier diagnosis, but it is demanding and requires specialised personnel. In an attempt to simplify the procedure, this study aimed to identify a new electrophysiological index, which might correlate with results obtained on standardised NCS in patients with long-standing type 2 diabetes.
Patients and methods: Medical records of type 2 diabetic patients evaluated for neuropathy by NCS were reviewed retrospectively. This analysis included 104 patients (50 men, 54 women) with a mean age of 67.1±5.5 years and mean diabetes duration of 13.1±2.7 years. NCS was performed on radial, ulnar, sural, and peroneal nerves. Neuropathy was defined as impaired NCS. Ratios of neurophysiological parameters from these nerves were calculated and each of them was compared with diagnosis of neuropathy.
Results: The sural sensory/radial motor amplitude ratio had the best combination of sensitivity (85%) and specificity (71%) for neuropathy. It also remained the strongest independent predictor of neuropathy in multivariate regression analysis: low levels of this ratio yielded an odds ratio of 7.7 for neuropathy.
Conclusions: The sural sensory/radial motor amplitude ratio has a high sensitivity and a moderately high specificity for the diagnosis of neuropathy, low levels being associated with a nearly eightfold increase in the risk for neuropathy. These results encourage further evaluation of this and other electrophysiological indices to enable wider availability of NCS.
Keywords: diabetes mellitus, diabetic peripheral neuropathy, nerve conduction study
Peripheral neuropathy is the most common neurological complication of diabetes mellitus1,2. It leads to a considerable increase in morbidity and is a cardinal factor in the pathogenesis of diabetic foot ulcer1–4. Clinical examination is the cornerstone of the diagnosis in everyday practice1,2. Nerve Conduction Study (NCS) significantly contributes to the detection of neuropathy1,5. More importantly, NCS enables earlier diagnosis of nerve injury6,7. This may be of value in an endeavour to prevent complications of neuropathy by early identification, intensification of glycaemic control and regular foot screening8,9.
However, NCS is not widely accessible and requires experienced personnel, which limits its utility as a screening test1,3. Therefore, automated electrophysiological devices have been developed, which may be used in the general diabetic clinic by staff with minimal training10–12. In the present study, in a similar effort to simplify NCS, we aimed to identify a new electrophysiological index showing good correlation with NCS impairment as evaluated by standardised NCS technique in patients with long-standing type 2 diabetes. This index should be based on electrophysiological examination of two nerves, which would be simpler and less time-demanding than the entire NCS.
Patients and methods
Medical records of type 2 diabetic patients evaluated for neuropathy by NCS were reviewed retrospectively. This analysis included 104 patients (50 men, 54 women) with mean age of 67.1±5.5 years and mean diabetes duration of 13.1±2.7 years attending the Outpatient Clinic of Obesity, Diabetes and Metabolism of the Second Department of Internal Medicine at Democritus University, Greece and the Diabetic Department of the University Hospital of Alexandroupolis, Greece. These patients had been referred to a tertiary care setting for evaluation of complications including examination by NCS.
Nerve conduction study (NCS) comprising conduction velocities, latencies and action potential amplitudes was carried out with a Nihon Kohden Neuropack Four Mini using temperature control and fixed distances for motor conduction. Motor conduction of the radial, ulnar and common and deep peroneal nerves, as well as sensory conduction of the radial, ulnar and sural nerves was recorded at non dominant limbs. Motor conduction was studied at the radial nerve by recording at extensor digitorum communis and stimulation a) 6 cm centrally, b) between brachioradialis and tendon of biceps, c) between coracobrachialis and medial edge of the triceps. Motor conduction was studied at the ulnar nerve by recording at abductor digiti minimi and stimulation a) 8cm centrally, at wrist b) below and c) above elbow. Motor conduction was assessed at the common and deep peroneal nerves by recording at extensor digitorum brevis and stimulation a) 7cm centrally b) below and c) above the head of fibula. Motor conduction in the aforementioned nerves was studied both centrally and distally, in order to exclude entrapment neuropathies. After exclusion of these conditions, distal motor nerve conduction was used for the assessment of diabetic neuropathy13,14. Sensory conduction was studied at the radial nerve by antidromic stimulation at the lateral edge of the radius in the distal forearm and recording at the back of the hand, between the first and second metacarpals. Sensory conduction was studied at the ulnar nerve by orthodromic stimulation at the fifth digit and recording at the wrist. Sensory conduction was studied at the sural nerve by antidromic stimulation along the posterior surface of the distal leg and recording behind the lateral malleolus.
All conduction velocities and action potential amplitudes were scored as 0= normal and 1= abnormal, using the mean reference value ± 2SD to define the normal range. Normal reference values were obtained by an examination of age-matched subjects from the population of the same area. The Total NCS score was defined as the sum of abnormal scores, and neuropathy was diagnosed in patients with a total NCS score ≥ 313.
Using the available data from NCS, ratios of neurophysiological parameters from various nerves were calculated. Each of them was compared with diagnosis of neuropathy based on the entire NCS. For the evaluation of the diagnostic significance of these ratios for neuropathy among diabetic patients, the area under the receiver operating characteristic (ROC) curve (AUC) was calculated. Sensitivity, specificity, positive and negative predictive values were calculated, while Cohen's kappa was used to assess agreement. The chi-square test was used to evaluate the potential associations between the presence or absence of neuropathy and the new indices. multivariate logistic regression analysis was employed to determine which ratios could be independent predictors of neuropathy. Odds ratios (OR) and 95% confidence intervals (CI) were estimated as the measure of association between the ratios of neurophysiological parameters with neuropathy. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 11.0 (SPSS, Inc., Chicago, IL, USA). All tests were two-tailed and statistical significance was defined at the 5% level (p< 0.05).
Results
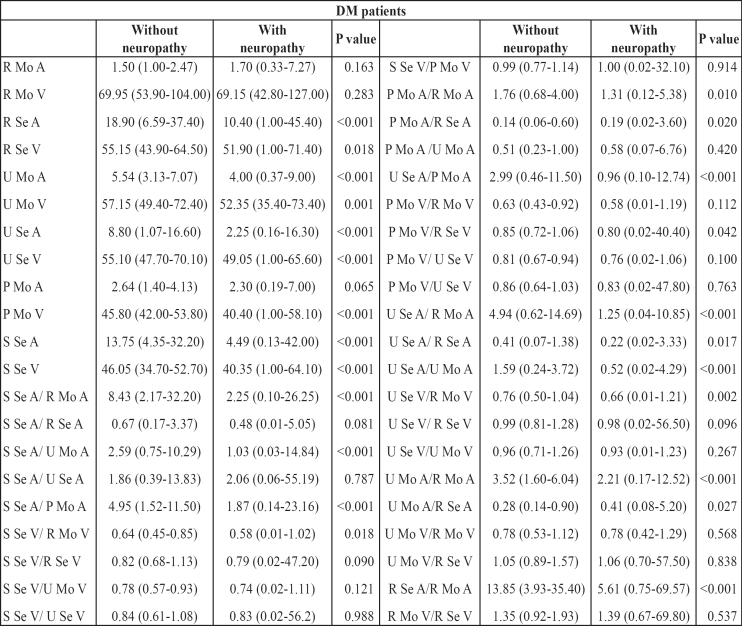
Overall, 71% (74 of 104 patients) had neuropathy. All electrophysiological parameters, when studied alone, were significantly higher among diabetic patients with neuropathy, except for radial motor amplitude, radial motor conduction velocity and peroneal motor amplitude, which did not differ significantly between the two groups (Table 1). The values of all ratios in relation to the presence or absence of neuropathy are also presented in Table 1. Fifteen of them showed significant differences between patients with and without neuropathy and were subjected to further analysis.
Table 1. The diagnostic indices in relation to the presence or absence of neuropathy.
R: Radial, S: Sural, U: Ulnar, P: Peroneal; Se: Sensory, Mo: Motor; A: Action Potential Amplitude, V: Conduction Velocity
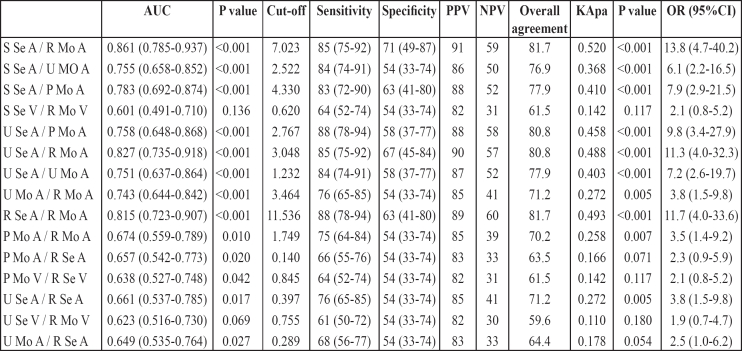
Table 2 shows the area under the receiver operating characteristic (ROC) curve (AUC) for these fifteen ratios. Analysis showed a superior performance of Sural sensory amplitude/Radial motor amplitude ratio (S Se A/ R Mo A) (AUC=0.861; 95% CI= 0.785-0.937; p<0.001), of Ulnar sensory amplitude/Radial motor amplitude ratio (U Se A/ R Mo A) (AUC=0.827; 95% CI= 0.735-0.918; p<0.001) and of Radial sensory amplitude/Radial motor amplitude ratio (R Se A/R Mo A) (AUC=0.815; 95% CI= 0.723-0.907; p<0.001). Five more ratios, Sural sensory amplitude/Peroneal motor amplitude ratio (S Se A/P Mo A), Sural sensory amplitude/Ulnar motor amplitude ratio (S Se A/U Mo A), Ulnar sensory amplitude/Peroneal motor amplitude ratio (U Se A/P Mo A), Ulnar sensory amplitude/Ulnar motor amplitude ratio (U Se A/U Mo A) and Ulnar motor amplitude/ Radial motor amplitude ratio (U Mo A/R Mo A) had AUC greater than 0.700, which indicates a high diagnostic significance. Clinically important cut-off points for all these diagnostic indices were also determined by the ROC curve analysis. These cut-offs for the fist three indices yielded very good sensitivities (85% to 88%), substantial specificities (63% to 71%) and positive predictive values (89% to 91%) and fair negative predictive values (57% to 60%). The overall agreement of patients' classification according to these indices with the initial clinical classification (with or without neuropathy) was over 80.0% (80.8% to 81.7%), while Cohen's kappa coefficient indicated substantial agreement, with kappa values ranging from 0.488 to 0.520 (all p< 0.001). Slightly inferior results were obtained for the other five ratios (Table 2).
Table 2. Characteristics of diagnostic indices.
R: Radial, S: Sural, U: Ulnar, P: Peroneal; Se: Sensory, Mo: Motor; A: Action Potential Amplitude, V: Conduction Velocity
Furthermore, logistic regression analysis revealed higher odd ratios for Sural sensory amplitude/Radial motor amplitude ratio (S Se A/R Mo A) (OR=13.8; 95% CI, 4.7 to 40.2), Radial sensory amplitude/Radial motor amplitude ratio (R Se A/ R Mo A) (OR=11.7; 95% CI, 4.0 to 33.6) and Ulnar sensory amplitude/Radial motor amplitude ratio (U Se A/R Mo A) (OR=11.3; 95% CI, 4.0 to 32.3) compared to odds ratios for the other indices.
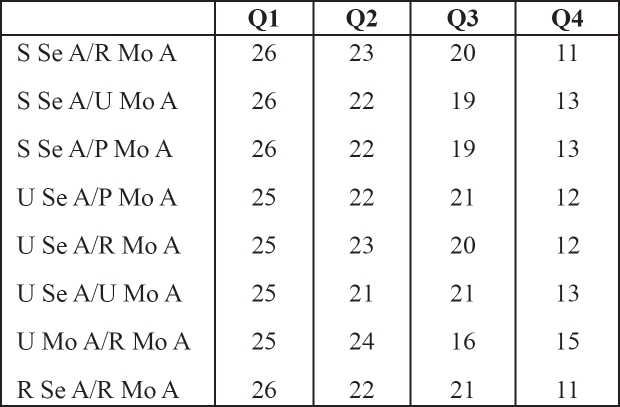
Correlation analysis between the eight ratios with high diagnostic significance and the presence or absence of neuropathy showed a significant negative association between the number of patients with neuropathy and quartiles of Sural sensory amplitude/Radial motor amplitude ratio (S Se A/R Mo A) (Kendal's tau–b=–0.447, p<0.001), quartiles of Sural sensory amplitude/Ulnar motor amplitude ratio (S Se A/ U Mo A) (Kendal's tau–b=–0.391, p<0.001), quartiles of Sural sensory amplitude/ Peroneal motor amplitude ratio (S Se A/ P Mo A) (Kendal's tau–b=–0.391, p<0.001), quartiles of Ulnar sensory amplitude/Peroneal motor amplitude ratio (U Se A/P Mo A) (Kendal's tau–b=–0.373, p<0.001), quartiles of Ulnar sensory amplitude/Radial motor amplitude ratio (U Se A/R Mo A) (Kendal's tau–b=–0.391, p<0.001), quartiles of Ulnar sensory amplitude/Ulnar motor amplitude ratio (U Se A/U Mo A) (Kendal's tau–b=–0.335, p<0.001), quartiles of Ulnar motor amplitude/Radial motor amplitude ratio (U Mo A/R Mo A) (Kendal's tau–b=–0.354, p<0.001), quartiles of Radial sensory amplitude/Radial motor amplitude ratio (R Se A/R Mo A) (Kendal's tau–b=–0.429, p<0.001). The frequency of neuropathy was higher in the lower quartiles of these ratios compared to the upper quartiles (Table 3).
Table 3. The frequency of neuropathy in the quartiles (Q1-Q4) of diagnostic indices.
R: Radial, S: Sural, U: Ulnar, P: Peroneal; Se: Sensory, Mo: Motor; A: Action Potential Amplitude, V: Conduction Velocity
Numbers in the body of the table represent patients
Finally, in multivariate logistic regression analysis, the Sural sensory amplitude/Radial motor amplitude ratio (S Se A/R Mo A), Ulnar sensory amplitude/Peroneal motor amplitude ratio (U Se A/P Mo A) and Radial sensory amplitude/ Radial motor amplitude ratio (R Se A/R Mo A) remained independent predictors of neuropathy; low levels of these ratios yielded odds ratios for neuropathy of 7.7 (95% CI, 2.2–27.5, p=0.002), 7.6 (95% CI, 2.1–27.7, p=0.002) and 5.2 (95% CI, 1.5–18.7, p=0.011) respectively.
Discussion
This study attempted to identify a potential new electrophysiological index that might correlate well with standardised NCS. Individual NCS parameters and the ratios between pairs of these parameters were examined. Most of these variables differed significantly between patients with and without neuropathy. Individual NCS parameters were not investigated further, because a single parameter (action potential amplitude or conduction velocity) in an individual nerve cannot exclude mononeuropathy of this particular nerve, and cannot, therefore, establish the diagnosis of polyneuropathy1,5,6,12. Fifteen ratios showed significant differences in relation to the presence or otherwise of neuropathy and were subjected to further analysis.
Analysis of the area under the receiver operating characteristic (ROC) curve (AUC) for these fifteen ratios showed a superior performance of three ratios: Sural sensory amplitude/Radial motor amplitude ratio, Ulnar sensory amplitude/Radial motor amplitude ratio and Radial sensory amplitude/Radial motor amplitude ratio. Of these, Radial sensory amplitude/Radial motor amplitude ratio could not be used for the diagnosis of polyneuropathy, because both parameters relate to one nerve. Sural sensory amplitude/Radial motor amplitude ratio (or simply: Sural sensory/Radial motor amplitude ratio) was the most useful diagnostic index, with 85% sensitivity, 71% specificity, 91% positive prognostic value, 59% negative prognostic value and the highest overall agreement (81.7%).
A low Sural sensory/Radial motor Amplitude ratio was accompanied by the highest odds ratio for neuropathy. More importantly, this new index remained the strongest independent predictor of neuropathy at multivariate regression analysis: low levels of this ratio yielded a nearly eightfold odds ratio of neuropathy.
In view of these correlations, it appears that the Sural sensory/Radial motor amplitude ratio was the index most closely associated with the presence of neuropathy, as documented by full NCS. This ratio was a strong predictor of neuropathy, with a high sensitivity and a moderately high specificity. Previous workers have also investigated electrophysiological indices of neuropathy15–18. Pastore and colleagues found that the Sural/Radial sensory amplitude ratio was associated with diabetes duration and showed the clearest correlation with the course of neuropathy, as well as being an early marker of nerve injury17. However, these observations were restricted to type 1 diabetic patients16. The Sural/Radial sensory amplitude ratio has been proposed as a sensitive, specific electrodiagnostic test for mild axonal polyneuropathy in general (including diabetes)18, but, more recently, its clinical value in the early detection of axonal polyneuropathies has been criticised16. Moreover, the Motor nerve conduction velocity/F-wave conduction velocity ratio has been suggested as a useful electrodiagnostic tool in the differential diagnosis of diabetic neuropathy from other causes (segmental demyelination, axonal neuropathy, alcoholic neuropathy and other causes)15. Our study design differs in terms of not searching an early marker or a tool for differential diagnosis. Instead, we sought to define a potential electrodiagnostic index showing good correlation with findings of classical NCS in patients with longstanding type 2 diabetes.
The limitations of this study include its retrospective design, as well as the absence of correlation with clinical data. However, the analysis was not aimed at defining an index showing high correlation with clinical neuropathy, but was designed to determine a potential electrophysiological index that would correlate well with classical NCS. The tertiary care setting, which accounts for the very high prevalence of neuropathy among our series, may pose a further limitation. Hence, caution is needed before applying these encouraging results to the general diabetic population. By no means should it be suggested that a simple electrophysiological index like the Sural sensory/ Radial motor amplitude ratio could replace the validated NCS. There is also no evidence that a simple electrophysiological index can estimate the risk for developing foot complications, as has been shown for NCS19.
The practical implications of the present study may be outlined as follows. Simple electrophysiological indices might prove useful in making NCS less time-demanding. Clearly, they still need a special nerve conduction laboratory and experienced personnel to be performed. Nonetheless, they permit a considerable reduction in time needed for the examination. Indeed, examination to calculate the Sural sensory/ Radial motor amplitude ratio would require approximately 10 minutes, while full NCS requires about 40 minutes. Even though a formal cost-effectiveness analysis was beyond the scope of the present work, it is plausible that this substantial reduction in the time needed for the procedure should enable the nerve conduction operators to review a far greater number of patients from general diabetic clinics, thereby rendering the laboratory more useful in clinical practice. Interestingly, this attempt at a simpler NCS (based on two parameters only for the Sural sensory/Radial motor amplitude ratio instead of the full examination) is in keeping with the recent approach towards an automated NCS to enable more widespread use10–12. At the end of the day, it is important to use resources more efficiently, in order to prevent underdiagnosis of diabetic neuropathy, which seems, currently, the case20. This ratio, with a high sensitivity and a moderately high specificity, appears promising and merits further evaluation.
In conclusion, this study has shown that the Sural sensory/ Radial motor amplitude ratio has a high sensitivity and a moderately high specificity for the diagnosis of NCS neuropathy. Indeed, low levels of this ratio were associated with a nearly eightfold increase in the risk for NCS neuropathy. These results encourage further evaluation of this and other electrophysiological indices to enable wider availability of NCS.
Footnotes
Conflicts of interest: None
Funding: None
References
- 1.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies. A statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 2.Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA. Diabetic neuropathy: an intensive review. Am J Health Syst Pharm. 2004;61:160–173. doi: 10.1093/ajhp/61.2.160. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJM. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47:1343–1353. doi: 10.1007/s00125-004-1463-y. [DOI] [PubMed] [Google Scholar]
- 4.Reiber GE, Vileikyte L, Boyko EJ, del AguilaM, Smith DG, Lavery LA, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22:157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 5.Valk GD, Nauta JJP, Strijers RLM, Bertelsmann FW. Clinical examination versus neurophysiological examination in the diagnosis of diabetic polyneuropathy. Diabet Med. 1992;9:716–721. doi: 10.1111/j.1464-5491.1992.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 6.Krarup C. An update on neurophysiological studies in neuropathy. Curr Opin Neurol. 2003;16:603–612. doi: 10.1097/01.wco.0000093104.34793.94. [DOI] [PubMed] [Google Scholar]
- 7.Rota E, Quadri R, Fanti E, Isoardo G, Poglio F, Tavella A, et al. Electrophysiological findings of peripheral neuropathy in newly diagnosed type II diabetes mellitus. J Peripher Nerv Syst. 2005;10:348–353. doi: 10.1111/j.1085-9489.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 8.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 10.Gozani SN, Fisher MA, Kong X, Megerian JT, Rutkove SB. Electrodiagnostic automation: principles and practice. Phys Med Rehabil Clin N Am. 2005;16:1015–1032. doi: 10.1016/j.pmr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kong X, Gozani SN, Hayes MT, Weinberg DH. NC-stat sensory nerve conduction studies in the median and ulnar nerves of symptomatic patients. Clin Neurophysiol. 2006;117:405–413. doi: 10.1016/j.clinph.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point-of-care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care. 2006;29:2023–2027. doi: 10.2337/dc08-0500. [DOI] [PubMed] [Google Scholar]
- 13.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract. 2002;54:115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 14.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 15.Ohgaki K, Nakano K, Shigeta H, et al. Ratio of motor nerve conduction velocity to F-wave conduction velocity in diabetic neuropathy. Diabetes Care. 1998;21:615–618. doi: 10.2337/diacare.21.4.615. [DOI] [PubMed] [Google Scholar]
- 16.Overbeek BU, van Alfen N, Bor JA, Zwarts MJ. Sural/radial nerve amplitude ratio: reference values in healthy subjects. Muscle Nerve. 2005;32:613–618. doi: 10.1002/mus.20421. [DOI] [PubMed] [Google Scholar]
- 17.Pastore C, Izura V, Geijo-Barrientos E, Dominguez JR. A comparison of electrophysiological tests for the early diagnosis of diabetic neuropathy. Muscle Nerve. 1999;22:1667–1673. doi: 10.1002/(sici)1097-4598(199912)22:12<1667::aid-mus8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Rutkove SB, Kothari MJ, Raynor EM, Levy ML, Fadic R, Nardin RA. Sural/radial amplitude ratio in the diagnosis of mild axonal polyneuropathy. Muscle Nerve. 1997;20:1236–1241. doi: 10.1002/(sici)1097-4598(199710)20:10<1236::aid-mus5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Carrington AL, Shaw JE, van Schie CHM, Abbott CA, Vileikyte L, Boulton AJM. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care. 2002;25:2010–2015. doi: 10.2337/diacare.25.11.2010. [DOI] [PubMed] [Google Scholar]
- 20.Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28:1480–1481. doi: 10.2337/diacare.28.6.1480. [DOI] [PubMed] [Google Scholar]