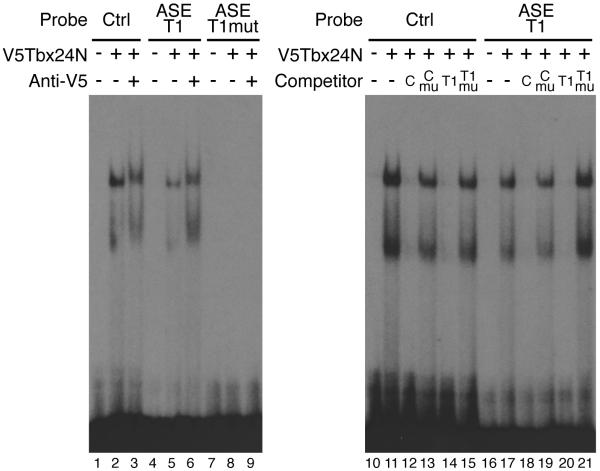
Fig.3. Identification of a Tbx binding site within the ASE.
A single Tbx24 binding site in the ASE was confirmed by EMSA (lanes 4-6). Control reactions contained unprogrammed lysate (lanes 1, 4 and 7). Tbx24N formed two complexes with the control T-box site and ASE-T1 probe (lanes 2 and 5). The faster migrating band is likely due to a truncated protein product in the in vitro translation reaction (data not shown). Specificity of binding was confirmed by supershifting complexes with an antibody to the V5 epitope (lanes 3 and 6). Mutation of the T-box in the ASE-T1 sequence abolished Tbx24N binding (lanes 7-9). Binding specificity was further confirmed by competition assays (lanes 10-21). Reciprocal competition was observed between the control and ASE-T1 oligonucleotides (lanes 12, 14, 18 and 20). Oligonucleotides containing mutated T-box sites were unable to compete (lanes 13, 15, 19 and 21). Unlabelled competitors were added at 100 fold molar excess.