Abstract
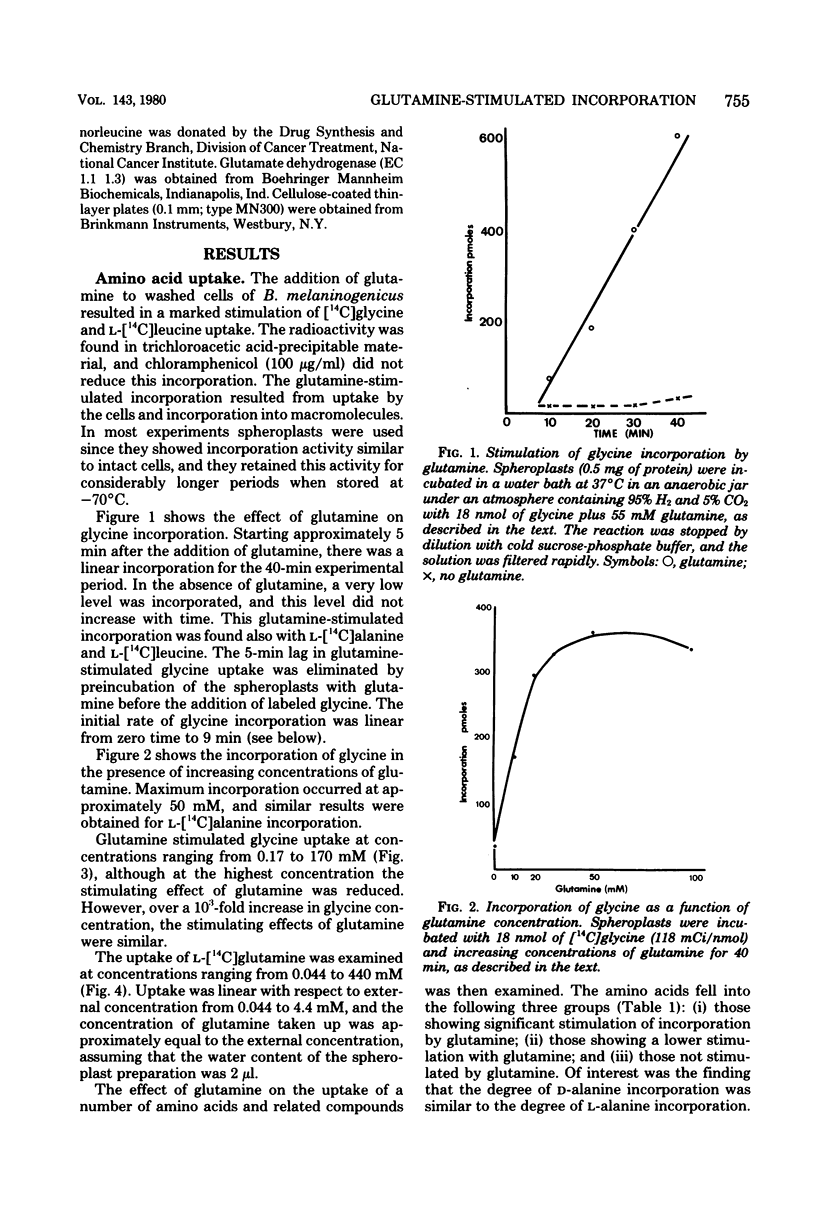
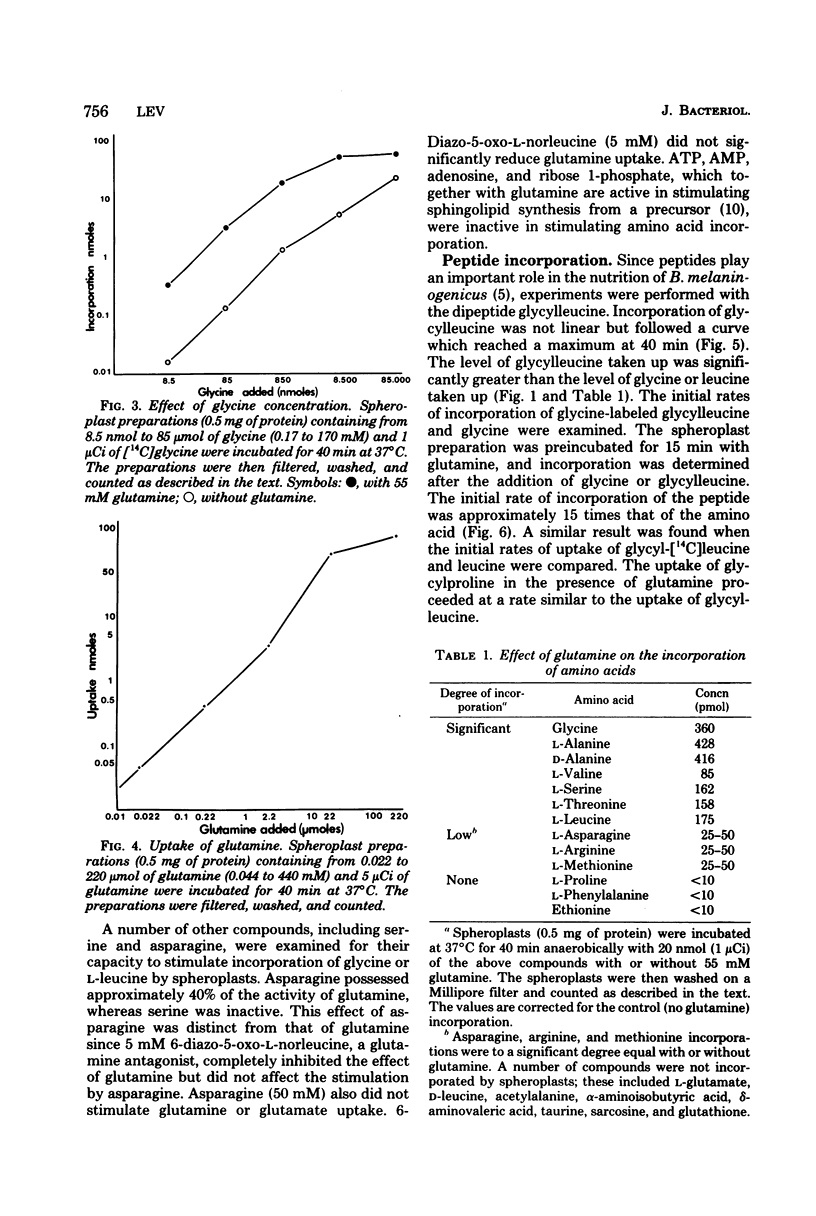
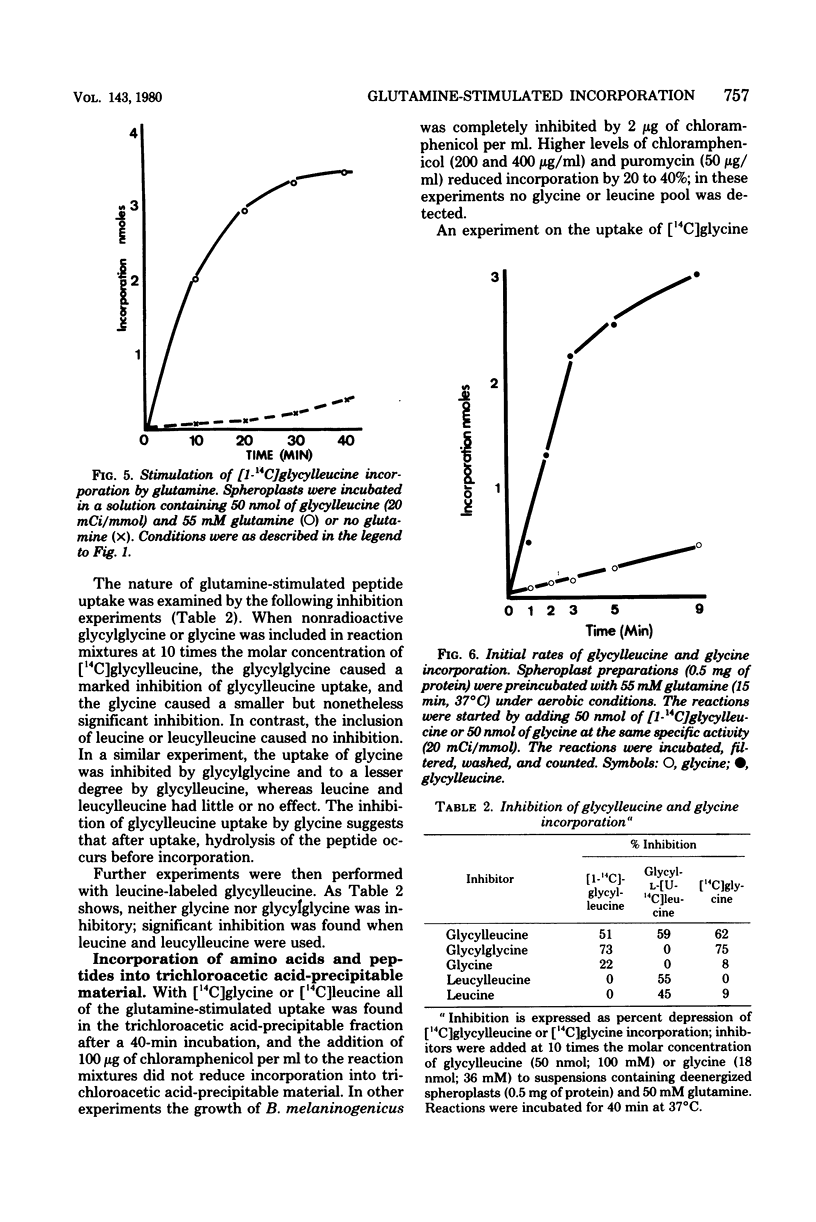
The uptake of a number of amino acids and dipeptides by cells and spheroplasts of Bacteroides melaninogenicus was stimulated by the presence of glutamine; 50 mM glutamine induced maximum uptake of glycine or alanine, and glutamine stimulated the uptake of glycine over a wide concentration range (0.17 to 170 mM). Glutamine stimulated the uptake of the dipeptides glycylleucine and glycylproline at significantly faster rates compared with glycine and leucine. The amino acids whose uptake was stimulated by glutamine were incorporated into trichloroacetic acid-precipitable material, and the inclusion of chloramphenicol or puromycin did not affect this incorporation. The uptake of glutamine by cells was concentration dependent. In contrast, in the absence of chloramphenicol 79% of the glutamine taken up by cells supplied with a high external concentration (4.4 mM) was trichloroacetic acid soluble. Glutamate and alpha-ketoglutarate were identified in the intracellular pool of glutamine-incubated spheroplasts. The amino acids and peptides were incorporated into cell envelope material, and a portion (30 to 50%) of the incorporated amino acids could be removed by trypsinization or treatment with papain. The effect of glutamine was depressed by inhibitors of energy metabolism, suggesting that glutamine-stimulated incorporation is an energy-mediated effect.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark V. L., Peterson D. E., Bernlohr R. W. Changes in free amino acid production and intracellular amino acid pools of Bacillus licheniformis as a function of culture age and growth media. J Bacteriol. 1972 Nov;112(2):715–725. doi: 10.1128/jb.112.2.715-725.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K., Heathcote J. G. The rapid resolution of naturally occurring amino acids by thin-layer chromatography. J Chromatogr. 1966 Sep;24(1):106–111. doi: 10.1016/s0021-9673(01)98107-5. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Boonstra J., De Vries W. Amino acid transport in membrane vesicles of obligately anaerobic Veillonella alcalescens. J Bacteriol. 1975 Apr;122(1):245–249. doi: 10.1128/jb.122.1.245-249.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Kaback H. R. Anaerobic transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3376–3381. doi: 10.1073/pnas.70.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M. Casamino acids enhance growth of Bacteroides melaninogenicus. J Bacteriol. 1977 Jan;129(1):562–563. doi: 10.1128/jb.129.1.562-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Keudell K. C., Milford A. F. Succinate as a growth factor for Bacteroides melaninogenicus. J Bacteriol. 1971 Oct;108(1):175–178. doi: 10.1128/jb.108.1.175-178.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Milford A. F. Apparatus for metabolic studies with anaerobes. Appl Microbiol. 1971 Mar;21(3):555–556. doi: 10.1128/am.21.3.555-556.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Milford A. F. Effect of vitamin K depletion and restoration on sphingolipid metabolism in Bacteroides melaninogenicus. J Lipid Res. 1972 May;13(3):364–370. [PubMed] [Google Scholar]
- Lev M., Milford A. F. Energy-dependent incorporation of sphingolipid precursors and fatty acids in Bacteriodes melaninogenicus. J Bacteriol. 1977 Apr;130(1):445–454. doi: 10.1128/jb.130.1.445-454.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Milford A. F. Role of nucleosides, 5-phosphoribosyl-1-pyrophosphate, and ribose 1-phosphate in the biosynthesis of phosphosphingolipids in Bacteroides melaninogenicus. Arch Biochem Biophys. 1978 Jan 15;185(1):82–87. doi: 10.1016/0003-9861(78)90146-7. [DOI] [PubMed] [Google Scholar]
- Lev M., Milford A. F. The 3-ketodihydrosphingosine synthetase of Bacteroides melaninogenicus: induction by vitamin K. Arch Biochem Biophys. 1973 Aug;157(2):500–508. doi: 10.1016/0003-9861(73)90668-1. [DOI] [PubMed] [Google Scholar]
- Lindley R. W., Delwiche E. A. Degradiation of alpha-ketoglutarate by Veillonella alcalescens. J Bacteriol. 1969 Apr;98(1):315–316. doi: 10.1128/jb.98.1.315-316.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Nuchamowitz Y. Biosynthesis of peptidoglycan in Pseudomonas aeruginosa. 1. The incorporation of peptidoglycan into the cell wall. Eur J Biochem. 1979 Mar;94(2):541–548. doi: 10.1111/j.1432-1033.1979.tb12923.x. [DOI] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewkow S., Ko H. L., Pulverer G. Nährstoffansprüche von Bacteroides melaninogenicus. Zentralbl Bakteriol Orig A. 1974;228(1):87–93. [PubMed] [Google Scholar]
- Vambutas V. K., Salton M. R. Incorporation of [14C]glycine into Micrococcus lysodeikticus membrane protein and effects of protein synthesis inhibitors. Biochim Biophys Acta. 1970 Mar 17;203(1):83–93. doi: 10.1016/0005-2736(70)90038-6. [DOI] [PubMed] [Google Scholar]


