Abstract
Objective
Manipulation under anesthesia (MUA) is an outpatient procedure that is performed to restore normal joint kinematics and musculoskeletal function. This article presents a case of a patient with idiopathic lumbar degenerative scoliosis who developed intractable pain as an adult and reports on the outcomes following a trial of MUA.
Clinical Features
A 59-year-old female patient presented to a chiropractic office with primary subjective symptoms of lower back and bilateral hip pain. Numerical pain rating scores were reported at 8 of 10 for the lower back and 9 of 10 for the sacroiliac joint/gluteal region. A disability score using a functional rating index demonstrated a score of 26 of 40 (or 64% disability). Over the preceding 5 years, the patient had tried a number of conservative therapies to relieve her pain without success.
Intervention and Outcome
The patient was evaluated for MUA. The patient was scheduled for a serial MUA over 3 days. Numerical pain rating scores 8 weeks after the MUA were 1 of 10 for the lower back and 3 of 10 for the sacroiliac joint. Her disability rating decreased to 11 of 40 (28%). Radiological improvements were also observed. These outcomes were maintained at 6-month follow-up.
Conclusion
Pain, functional, and radiographic outcomes demonstrated improvements immediately following treatment for this patient.
Key indexing terms: Anesthesia, Manipulation, Spinal, Scoliosis, Chiropractic
Introduction
Manipulation under anesthesia (MUA) is an outpatient procedure that is performed to restore normal joint kinematics and musculoskeletal function. Manipulation under anesthesia for spinal conditions has been performed at least as early as 1930.1 In 1952, Siehl and Bradford2 reported their results of 87 patients treated for lumbar disk herniation. Since that time, several case reports have been published on hundreds of patients on the clinical effectiveness of MUA3-10 for chronic musculoskeletal pain and dysfunction. For example, in 1955, Mensor3 reported results in 205 cases of lumbar intervertebral disk syndrome, wherein 51% of patients obtained a “good” or “excellent” result. In the largest study to date, Siehl4 published his results of 666 cases of nonspecific back pain. About 90% of these patients received a “good” or “fair” result. Another 1986 study by Krumhansl and Nowacek6 showed that 75% of 171 patients rated their improvements as “much improved” or “cured.”
More recently, a study by West et al9 evaluated 177 patients 6 months and 2 years following spinal MUA. They found that pain medication use among patients after MUA was reduced by 58%; cervical and lumbar spine range-of-motion increased 47% and 83%, respectively; and visual analog scale scores decreased 62% in the cervical spine and 60% in the lumbar spine. These results were maintained at the 2-year follow-up.
The clinical indications for MUA are quite comprehensive. These indications have been predominantly based on anecdotal evidence. However, there tends to be universal agreement on these indications. A list of the indications and contraindications can be found in Fig 1. This figure has been adapted from the curricula of 4 different postgraduate MUA courses.11-14
Fig 1.
Indications and contraindications for MUA
| Indications |
| • Disk herniation/prolapse/protrusion/bulge |
| • Joint or spinal ankylosis |
| • Failed low back surgery |
| • Nonsurgical conditions that have reached maximum medical improvement |
| • Nonresponsive muscle contraction |
| • Whiplash-associated disorders |
| • Compression syndromes with nonosteophytic entrapment |
| • Pain interfering with daily activities or sleep |
| Contraindications |
| • Malignancy |
| • Bone metastasis |
| • Bony tuberculosis |
| • Acute fracture |
| • Acute autoimmune arthritides |
| • Acute gout |
| • Venereal spinal or joint infiltration |
| • Advanced osteoporosis |
| • Osteomyelitis |
| • Septicemia |
| • Spondylolysis |
| • Active spondylolisthesis |
Scoliosis is a condition that can cause chronic back pain in adulthood.15 Nonsurgical treatment of scoliosis is typically designed to treat the pain and symptoms associated with scoliosis, rather than correcting the spinal curvature. Among these treatments includes pilates, chiropractic manipulation, and massage therapy, all of whose individual efficacy remains largely unknown.16,17 The first line of conventional treatment of idiopathic scoliosis is usually observation, when the Cobb angle is less than 25° to 30°.18 Many of these spinal curvatures never progress beyond this observational threshold. Therefore, a certain portion of patients with idiopathic scoliosis, even when diagnosed as a juvenile or adolescent, never end up having treatment of any kind beyond observation. However, scoliotic curvatures that remain into adulthood have a linear rate at which they progress.19
Several case reports have been published about the clinical utility of MUA for chronic musculoskeletal pain and dysfunction. However, MUA has not yet been reported for use on a patient with adult degenerative scoliosis causing intractable pain that had been only partially responsive to many other conservative therapies. This article presents a case of a patient with idiopathic lumbar degenerative scoliosis who developed intractable pain as an adult, wherein treatment as an adolescent was not sought. We report on the patient's history of her scoliosis, past treatment in adulthood, and her outcomes following a trial of MUA. The patient gave both verbal consent and HIPAA-approved written consent to have her results published.
Case report
Clinical features
A 59-year-old female patient presented to our office with a primary complaint of lower back and bilateral hip pain. The lower back pain was described as an intense ache that was unchanged by varying postures or movements. Her pain was reduced in the morning when arising from bed and gradually increased throughout the day. The pain was located bilaterally at approximately the L2-L5 paraspinal levels, with the left side being noticeably worse than the right. She also reported bilateral hip pain with radiation into the right sacroiliac joint and gluteal region. Although both hips were painful during movement, only the right hip was accompanied by radiating pain into the posterior thigh and knee. Numerical pain rating scores (patient was asked what her pain was at that moment) were reported at 8 of 10 for the lower back and 9 of 10 for the sacroiliac joint/gluteal region. Lumbar spine flexion and extension were reduced by 66% and 75%, respectively; right lumbar lateral flexion was reduced by 80%; and left and right hip mobility was reduced in all planes. Palpatory tenderness was present over the right sacroiliac joint and piriformis muscle, as well as over the lumbar multifidis and quadratus lumborum bilaterally. The disability score using a functional rating index was 26 of 40 (or 64% disability). Radiographically, the patient's spinal curvature was located in the thoracolumbar spine, with a T11 upper-end vertebra and L4 lower-end vertebra, with a 49° Cobb angle. Nash-Moe rotation of the apical L1 vertebra was 3+, with an apical deviation of 39 mm.
With one exception, her chief complaints could not be aggravated by orthopedic testing of the lumbar spine and hip. However, a standing stork test demonstrated a sharp increase in pain in the right sacroiliac joint when standing on the right leg with the lower spine and pelvis slightly hyperextended. Based on the history and examination, the patient was diagnosed with right sacroiliitis and myofascitis of the right gluteal and erector spinae muscles.
The patient's medical history included hysterectomy, with follow-up abdominal surgery to remove fibroadhesions. The patient had also been diagnosed with bilateral carpal tunnel syndrome via electrodiagnostic testing. No evidence of lumbar radiculopathy was found. Over the preceding 5 years, the patient had tried a number of conservative therapies to relieve her chief complaints. These included chiropractic manipulation weekly for 8 months, 12 consecutive weeks of physical therapy, 3 corticosteroid injections into the sacroiliac joints 6 to 8 weeks apart, an epidural steroid injection in the lumbar spine, massage therapy, nonsteroidal anti-inflammatory drugs, muscle relaxants, and opioid medications.
Given the treatment history of the patient, including surgical history of fibroadhesions, the examining physician (MNS) recommended the MUA procedure. The patient was scheduled for preanesthesia testing. To determine if the patient was a suitable candidate for the anesthesia used during the MUA procedure, the patient was referred for the following standard preanesthesia testing: a Chem 18 panel, including prothrombin, partial thromboplastin time, and the international normalized ratio; a chest radiograph (because the patient was older than 50 years); and an electrocardiogram due to age and positive cardiovascular history (hyperlipidemia, hypertension). Previous electrodiagnostic studies of the upper and lower extremities taken within the prior year were used to determine the patient's prognosis with MUA.
Subjective and objective clinical determinants for MUA candidacy have been outlined as follows20:
-
1.
Neuromusculoskeletal complaints that have only partially responded to manipulation, yet manipulation is still the therapy of choice.
-
2.
Neuromusculoskeletal complaints that normally respond to manipulation, but the complaint is too painful to allow manipulative intervention.
-
3.
Patients with chronic conditions that have previously been treated to maximum medical improvement, but the patient continues to experience regular exacerbations.
-
4.
Patients who have been treated for 2 to 8 weeks but continue to maintain a pain threshold that disallows manipulative intervention.
-
5.
Patients who cannot control voluntary muscle contraction during manipulation or are causing manipulative therapy to be prolonged. Manipulation under anesthesia would reduce the patient's treatment period.
-
6.
Patients who are candidates for manipulation but the extent of the presenting injury has limited the effectiveness of in-office manipulation where more joint movement is necessary to produce expected clinical results.
-
7.
Patients who are considered disk surgery patients but fall within the parameters of MUA, which may be an alternative or interim step and may be useful as either a therapeutic or diagnostic tool in determining the prognosis of the patient's care.
-
8.
Patients who are candidates for manipulation, but because of restrictive adhesions causing articular fixation, are responding only minimally to clinical conservative care.
Based upon one of the authors' (MNS) initial history of the patient, she felt that the patient was an MUA candidate based upon reasons 1, 5, and 8 above.
Intervention and outcome
Once MUA candidacy was approved, the patient was scheduled for a serial MUA over 3 days. The procedures were performed at an ambulatory surgical center in West Bloomfield, MI. Before the MUA, the patient was given robinol intravenously to decrease oral secretions and torodol to minimize postprocedural discomfort. Once the anesthesiologist was ready, the patient was taken to the surgical suite where she was assisted into a deep conscious sedation using propofol. The patient was not responsive during the MUA. The MUA techniques used consisted of a combination of passive stretching and low-velocity/moderate-amplitude manipulative techniques for treating dysfunctional articulations. These techniques are modified to accommodate varying body types, conditions, as well as anatomical variations. These modifications are essential to prevent joint damage, ligamentous instability, and/or neurologic compromise. Passive stretching was combined with distraction to increase range of motion while decreasing fibroadhesions. Passive stretching was performed to each joint's elastic end range of motion and held there for approximately 15 to 20 seconds. Muscular trigger points were treated with percussive techniques or myofascial release techniques at the time of passive stretching to overcome any muscular resistance.
Subsequent to the stretches, low-velocity/moderate-amplitude articular manipulation was delivered to the dysfunctional spinopelvic segments. In this case, MUA techniques were performed on the thoracic spine, lumbar spine, pelvis, and hip joints bilaterally. These techniques are thoroughly illustrated by Gordon.20 All of the procedures were performed by both of the authors in tandem. Because the patient was nonresponsive, one physician performed the techniques, whereas the other stabilized the patient. Following the conclusion of the MUA, the patient was taken by hospital bed to recovery and was discharged without any intra- or postoperative complications. The patient was instructed to relax for the rest of the day and report back for days 2 and 3 of the serial MUA. Before days 2 and 3 of the MUA, the patient was assessed to document any subjective and objective improvements following the first MUA procedure. Subsequent MUAs are not administered if functional or subjective improvements are not observed, or if the patient improves 80% or more after the first MUA. However, a second and third MUA is scheduled if the patient makes an approximately 50% to 70% improvement following the prior MUA.20 Each of the procedures discussed above was repeated on both days 2 and 3. When the patient was discharged on the third day, the author (MNS) prescribed 600 mg ibuprofen every 6 hours to mitigate the inflammatory response, thus inhibiting the reformation of fibroadhesions around the affected joint structures.
Following the MUA on the third day, the patient reported later in the afternoon for her first session of post-MUA rehabilitation therapy. This therapy was prescribed for 3 visits weekly for 8 weeks. During this visit, the patient again completed a functional rating index, which resulted in a score of 20 of 40 (or 50% disability). She stated that her pain level overall was 6 of 10 for both the lower back and hips. Initially, her first week of post-MUA therapy consisted mainly of passive procedures, such as cryotherapy, massage therapy, light assisted stretching, and intersegmental traction. She also received vibration inversion therapy to counteract the gravitational stress on her spinal curvature for approximately 10 minutes (Fig 2). As the patient progressed with care, she was also given an external weighting system developed by Pettibon (Chehalis, WA; patent #6,788,968; Fig 3).
Fig 2.
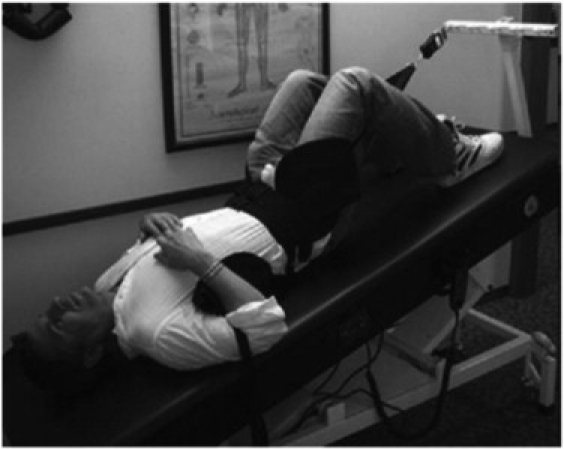
Photograph of vibration inversion therapy. The patient was inverted approximately 20° and vibrated at approximately 25 Hz. This procedure was performed at every office visit for the entire 8-week trial.
Fig 3.

Image of external weighting system designed to facilitate correction of the thoracolumbar curvature while at home. She was instructed to wear these weights at home daily for 15 minutes beginning in week 3 of her care. These weights were specifically configured for her based upon her existing spinal structure, and she was radiographed while wearing the weights to ensure correct placement.
After 8 weeks of post-MUA therapy, the patient was again asked to complete a functional rating index and provide a numerical pain rating; and her ranges of motion in her lumbar spine and hips were observed. Her functional rating index score was 11 of 40, or 28% disability rating, whereas her pain scores were rated as 1 of 10 for the low back and 3 of 10 for the hips. Her lumbar flexion and extension both increased approximately 60%, whereas her hips significantly improved in all planes of motion. The patient verbalized that she was very pleased with her results. The patient's thoracolumbar spinal curvature improved on postintervention radiograph to a 38° Cobb angle and an apical deviation of 31 mm (Fig 4). Upon release from therapy, the patient was instructed on specific home care stretches and exercises that had been previously performed in the clinic setting. She was told to perform these exercises twice a day, 3 days a week. All of the above values were maintained 6 months following her release from post-MUA therapy, including continued improvement in her functional rating index score (10/40 or 25% disability); lumbar flexion increased an additional 8%; and bilateral hip pain was rated at 2 of 10.
Fig 4.
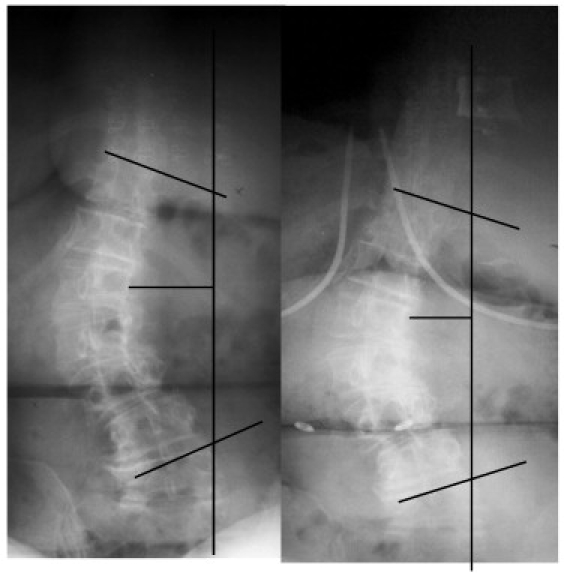
Pre- and postintervention films showing Nash-Moe rotation of the apical L1 vertebra reduced to 2+.
Discussion
Previously reported data suggest that more than two thirds of adult scoliosis cases progress after skeletal maturity.21 Whereas surgical management has been well documented,22 conservative options for adult scoliosis are relatively scant.23-27 Therefore, any and all possible conservative treatment options should be investigated so that adult scoliosis patients have more care choices.
The incidence of scoliosis is reportedly 2% to 3% of the population.28 We mention this because in a review by Kohlbeck and Haldeman,29 they cite a total of 1525 peer-reviewed MUA cases. These patients were treated predominantly for chronic spine conditions not unlike our patient here. Applying this scoliosis incidence to these 1525 cases means that potentially 30 to 45 of these patients had scoliosis at the time of their MUA. However, we could not find any mention of scoliosis in our review of these articles. It may be prudent in the future to look at how different types of comorbid spinal pain syndromes may impact, or be impacted by, scoliosis. In our experience, because of the chronicity of our patient's complaints and the long history of scoliosis, we believed that an extremely protracted treatment plan would be required to significantly affect her scoliotic curvature, based upon research using the rehabilitative modalities outlined here.23,24 Therefore, our decision to use MUA for this patient was at least partially based on ultimately wanting a shorter treatment duration.
Using MUA in this particular case was based on the lack of flexibility and resultant pain found in the spine and hips. These symptoms had been largely unresponsive to other conservative approaches, including conventional chiropractic manipulation. Other researchers have reported that the addition of anesthesia can significantly increase the flexibility of scoliotic curvatures, which can be a better predictor of surgical outcomes.30 Our findings with this patient, as well as those findings of Ibrahim et al,30 suggest that flexibility can be improved once normal reflexive muscle response is muted by the introduction of anesthesia.
Historically, MUA has maintained a low rate of complications. Kohlbeck and Haldeman29 identified a complication rate of 0.7% in their 2002 review. This rate compares favorably against injection therapy, for example, which has a range of 1.64% to 10% depending upon the anatomical region involved.31,32 This is an important statistic for patients in determining which therapies they would decide to pursue.
By virtue of being a case report, we can only draw limited conclusions as a result of the study design. Following a course of 3 serial manipulations under anesthesia and 8 weeks of post-MUA therapy, the patient reported improved outcomes in activities of daily living and pain. Comparative radiographs demonstrated an improved spinal coronal balance and reduced Cobb angle measurement. Despite these demonstrated improvements, it is impossible to say for certain which specific modality(ies), if any, accounted for the observed improvements.
Because conventional treatments for scoliosis are typically prescribed for adolescents and not adults, adult patients may often seek unconventional or alternative medicine treatment methods. The patient presented in this case study had pain that we believed ultimately arose from the presence of her lumbar degenerative scoliosis. This may be why her symptoms were largely unresponsive to other nonsurgical therapies. Our own theory is that the presence of chronic muscular contraction and ligamentous shortening in the concavities of scoliotic curvatures may be too much resistance for nonsurgical therapies to overcome. Therefore, we believe that the addition of MUA to this patient's total treatment plan allowed us to mobilize regions of her spine and pelvis, which was not successful by other means. Because the intensity and frequency of her symptoms would have likely interfered with the rehabilitation system used in this case, MUA was a critical piece of the treatment puzzle for this patient to achieve her good clinical outcome. We believe that adult patients with chronic degenerative scoliosis may benefit from serial MUA if their symptoms preclude performance of a comprehensive and moderately aggressive spinal rehabilitation program. This would include instances where reducing the scoliotic Cobb angle was not necessarily a goal of treatment.
Conclusion
An adult patient with degenerative lumbar levoscoliosis was treated conservatively using MUA followed by 8 weeks of multimodal rehabilitation therapy. Pain, functional, and radiographic outcomes all demonstrated improvements immediately following treatment. These improvements were maintained 6 months following the conclusion of therapy. This case adds to the body of literature illustrating the use of MUA in cases where previous therapies were tried unsatisfactorily. Adults with scoliosis, who have few conservative treatment options to choose from, may be helped symptomatically and/or functionally by procedures like MUA that is designed to increase articular range of motion in a short period. Manipulation under anesthesia was performed on this adult patient with moderate degenerative scoliosis without any intra- or postoperative complications.
Funding sources and potential conflicts of interest
No funding sources or conflicts of interest were reported for this study.
Contributor Information
Mark W. Morningstar, Email: drmark2star@yahoo.com.
Megan N. Strauchman, Email: drmstrauchman@yahoo.com.
References
- 1.Riches E.W. End-results of manipulation of the back. Lancet. 1930;1:957–960. [Google Scholar]
- 2.Siehl D., Bradford W.G. Manipulation of the low back under general anesthesia. J Am Osteopath Assoc. 1952;52:239–242. [PubMed] [Google Scholar]
- 3.Mensor M.C. Non-operative treatment, including manipulation, for lumbar intervertebral disc syndrome. J Bone J Surg. 1955;37A:925–936. [PubMed] [Google Scholar]
- 4.Siehl D. Manipulation of the spine under general anesthesia. J Am Osteopath Assoc. 1963;62:881–887. [PubMed] [Google Scholar]
- 5.Morey L.W. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116–127. [PubMed] [Google Scholar]
- 6.Krumhansl B.R., Nowacek C.J. Manipulation under anesthesia. In: Grieve G.P., editor. Modern manual therapy of the vertebral column. Churchill Livingstone; Edinburgh: 1986. pp. 777–786. [Google Scholar]
- 7.Greenman P.E. Manipulation with the patient under anesthesia. J Am Osteopath Assoc. 1992;92:1159–1169. [PubMed] [Google Scholar]
- 8.Cremata E., Collins S., Clauson W., Solinger A.B., Roberts E.S. Manipulation under anesthesia: a report of 4 cases. J Manipulative Physiol Ther. 2005;28:526–533. doi: 10.1016/j.jmpt.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 9.West D.T., Mathews R.S., Miller M.R., Kent G.M. Effective management of spinal pain in one hundred seventy seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299–308. doi: 10.1016/s0161-4754(99)70062-x. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri N.F., Smoyak S. Chronic low back pain: a study of the effects of manipulation under anesthesia. J Manipulative Physiol Ther. 2002;25:E8–17. doi: 10.1067/mmt.2002.127072. [DOI] [PubMed] [Google Scholar]
- 11.Gordon R. Lincoln School of Postgraduate Education; Lombard (Ill): 2001. Manipulation under anesthesia course notes. [Google Scholar]
- 12.Francis R. Texas Chiropractic College; Pasadena (Tex): 2000. Manipulation under anesthesia course notes. [Google Scholar]
- 13.Cerf J., Salamone J. New York Chiropractic College; Seneca Falls (NY): 2008. Manipulation under anesthesia course notes. [Google Scholar]
- 14.Kelly W. College of Chiropractic, University of Bridgeport; Bridgeport (Conn): 2006. Manipulation under anesthesia course notes. [Google Scholar]
- 15.Weinstein S.L., Dolan L.A., Spratt K.F., Peterson K.K., Spoonamore M.J., Ponseti I.V. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. 2003;289:559–567. doi: 10.1001/jama.289.5.559. [DOI] [PubMed] [Google Scholar]
- 16.Blum C.L. Chiropractic and pilates therapy for the treatment of adult scoliosis. J Manipulative Physiol Ther. 2002;25:E3. doi: 10.1067/mmt.2002.123336. [DOI] [PubMed] [Google Scholar]
- 17.Romano M., Negrini S. Manual therapy as a conservative treatment for adolescent idiopathic scoliosis: a systematic review. Scoliosis. 2008;3:2. doi: 10.1186/1748-7161-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greiner K.A. Adolescent idiopathic scoliosis: radiologic decision-making. Am Fam Physician. 2002;65:1817–1822. [PubMed] [Google Scholar]
- 19.Marty-Poumarat C., Scattin L., Marpeau M., Garreau de Loubresse C., Aegerter P. Natural history of progressive adult scoliosis. Spine. 2007;32:1227–1234. doi: 10.1097/01.brs.0000263328.89135.a6. [DOI] [PubMed] [Google Scholar]
- 20.Gordon R.C., editor. Manipulation under anesthesia: concepts in theory and application. CRC Press Taylor & Francis Group; Boca Raton (Fla): 2005. [Google Scholar]
- 21.Weinstein S.L. Natural history. Spine. 1999;24:2592–2600. doi: 10.1097/00007632-199912150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–948. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 23.Morningstar M.W., Woggon D., Lawrence G. Scoliosis treatment using a combination of manipulative and rehabilitative therapy: a retrospective case series. BMC Musculoskelet Disord. 2004;5:32. doi: 10.1186/1471-2474-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morningstar M.W., Joy T. Scoliosis treatment using spinal manipulation and the Pettibon weighting system: a summary of three atypical presentations. Chiropr Osteopath. 2006;14:1. doi: 10.1186/1746-1340-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss H.R. Influence of an in-patient exercise program on scoliotic curve. Ital J Orthop Traumatol. 1992;18:395–406. [PubMed] [Google Scholar]
- 26.Tarola G.A. Manipulation for the control of back pain and curve progression in patients with skeletally mature idiopathic scoliosis: two cases. J Manipulative Physiol Ther. 1994;17:253–257. [PubMed] [Google Scholar]
- 27.Negrini A., Parzini S., Negrini M.G., Romano M., Atanasio S., Zaina F. Adult scoliosis can be reduced through specific SEAS exercises: a case report. Scoliosis. 2008;3:20. doi: 10.1186/1748-7161-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawes M.C. West Press; Tucson (Ariz): 2002. Scoliosis and the human spine. [Google Scholar]
- 29.Kohlbeck F.J., Haldeman S. Medication-assisted manipulation. Spine J. 2002;2:288–302. doi: 10.1016/s1529-9430(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim T., Gabbar O.A., El-Abed K., Hutchinson M.J., Nelson I.W. The value of radiographs obtained during forced traction under general anesthesia in predicting flexibility in idiopathic scoliosis with Cobb angles exceeding 60 degrees. J Bone J Surg Br. 2008;90:1473–1476. doi: 10.1302/0301-620X.90B11.20690. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra G., Abbasi A., Rhee M. Complications of transforaminal cervical epidural steroid injections. Spine. 2009;34:731–739. doi: 10.1097/BRS.0b013e318194e247. [DOI] [PubMed] [Google Scholar]
- 32.Bhargava A., DePalma M.J., Ludwig S., Gelb D., Slipman C.W. Injection therapy for lumbar radiculopathy. Curr Opin Orthop. 2005;16:152–157. [Google Scholar]


