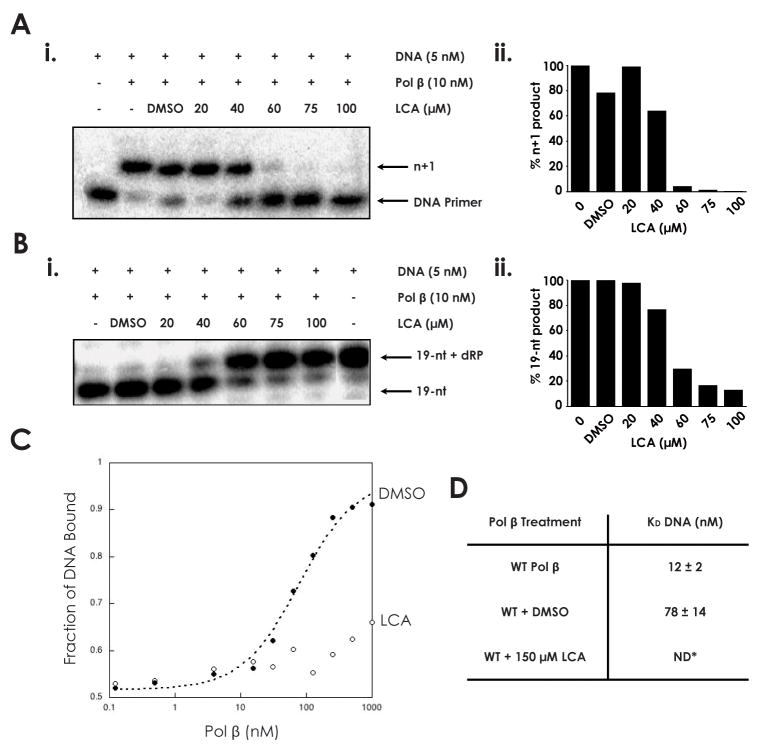
Fig. 1.
Inhibition of DNA polymerase β by lithocholic acid (A) i. 45AG DNA substrate (Table 1) was incubated with pol β and increasing concentrations of LCA to measure DNA polymerization activity. Formation of n+1 product was analyzed via gel electrophoresis. ii. n+1 polymerization product obtained at each LCA concentration, normalized to untreated sample. (B) i. LPSD DNA substrate (Table 1) was incubated with pol β and increasing concentrations of LCA to measure dRP lyase activity. Formation of 19-nt (no dRP) product was analyzed via gel electrophoresis. ii. 19-nt product obtained at each LCA concentration, normalized to untreated sample. (C) The binding of pol β to a DNA substrate containing a 1-bp gap was analyzed in the presence of DMSO and 150 μM LCA. (D) DNA dissociation constants (KD DNA) of wildtype pol β (33), pol β with DMSO, and pol β with 150 μM LCA, as measured from binding assay. ND: not determinable.