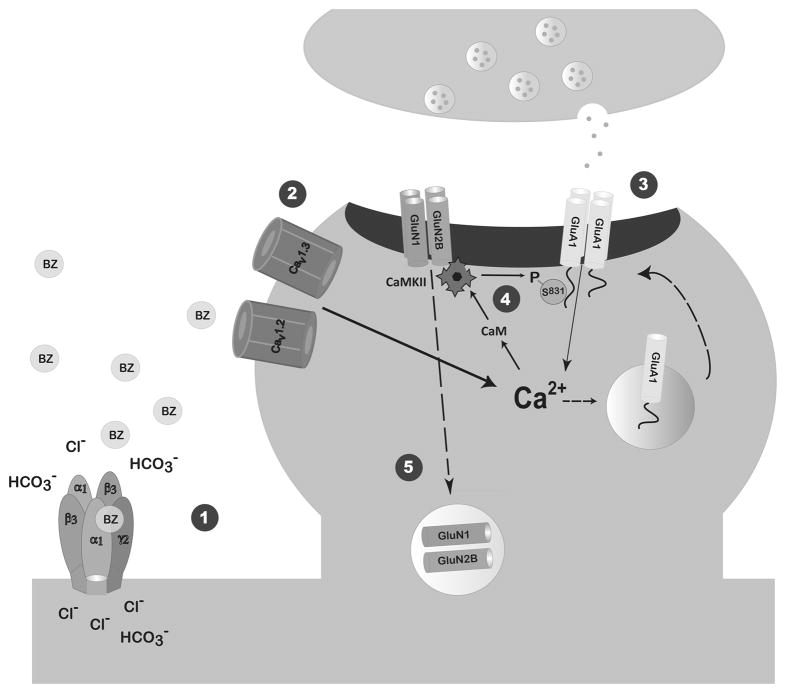
Figure 6. Benzodiazepine Withdrawal Model.
We propose that 1: persistent benzodiazepine enhancement of the inhibitory GABAA receptor leads to a bicarbonate ion-dependent GABA-mediated depolarization (Zeng and Tietz, 2000) through an as yet undefined mechanism and 2: a doubling of L-type voltage-gated calcium channel (L-VGCC) current density (Xiang et al., 2008). Since both AMPAR potentiation and BZ withdrawal-anxiety were prevented by systemic pre-injection of either an AMPAR or a L-VGCC antagonist, but not an NMDAR antagonist (Van Sickle et al., 2004, Xiang and Tietz, 2007), we postulate that the increased Ca2+ influx through L-VGCCs mediates 3: insertion of AMPAR GluA1 homomers and increased AMPAR current amplitude at CA1 synapses of FZP-withdrawn rats (Song et al., 2007, Shen et al., 2010). Increased Ca2+ influx largely through L-VGCCs, but also possibly through Ca2+-permeable GluA1 homomers leads to 4: CaMKII-mediated Ser831 phosphorylation of GluA1 homomers and increased AMPAR current conductance 2 days after drug withdrawal (Shen et al., 2009, 2010). All dashed arrows represent as yet undefined mechanisms. 5: NMDAR function and expression is decreased secondary to AMPAR potentiation. Data from the current and earlier studies (Van Sickle et al., 2002, Shen et al., 2008) suggest that GluN2B-containing NMDARs in the CA1 synapse are regulated by decreased insertion and/or increased removal from the synapse. Decreased synaptic GluN2B-containing NMDARs may serve as a physiological brake to mitigate CA1 neuron hyperexcitability and BZ withdrawal-anxiety.