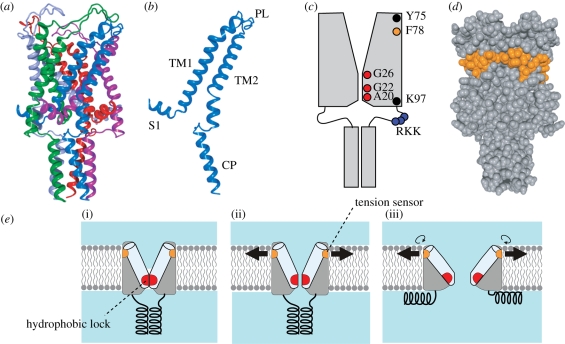
Figure 3.
Structure and function of the MscL mechanosensitive channel. (a) Crystal structure of M. tuberculosis MscL (PDB accession number: 2OAR). Each subunit is in a different colour. (b) Structure of single subunit of TbMscL. Shown are N-terminal helix (S1), the first and second transmembrane helices (TM1 and TM2), periplasmic loop (PL) and cytoplasmic helix (CP). (c) Positions of important residues in the schematic EcMscL. The pore is constricted between A20 and G26. TM2 is immersed in lipid bilayer between Y75 and K97. Gating threshold changes with the hydrophilicity of G22 and mechanosensitivity is lost on hydrophilic substitution of Y78. Charged residues RKK are involved in pH sensitivity and oligomer assembly. (d) Hydrophobic residues that hamper mechanosensiticity on asparagine substitution. Residues corresponding to EcMscL are indicated in orange in the space fill model of TbMscL. (e) Gating model of MscL. The closed structure is stabilized by the hydrophobic lock of the gate (i). The membrane tension perceived by the tension sensor opens the gate, which results in the exposure of the hydrophobic lock to water (ii). On full opening, the cytoplasmic helices are disassembled and the hydrophobic lock is buried in the protein interior by the rotation of TM1 (iii).