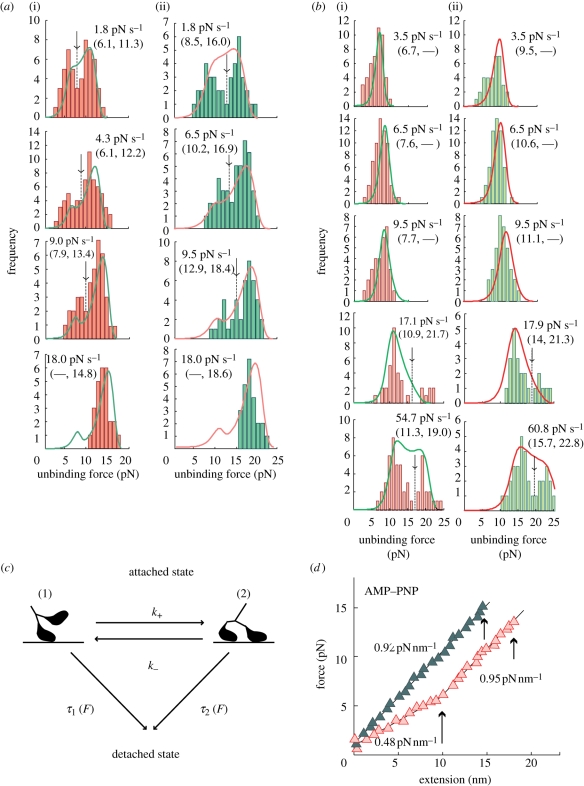
Figure 4.
Binding mode of kinesin depends on loading rate. (a) Loading rate dependence of the unbinding force distribution in the AMP–PNP state. Unbinding force was measured by loading towards either the plus end (a(i)) or the minus end (a(ii)). (b) Loading rate dependence of the unbinding force distribution in the nucleotide-free state. Unbinding force was measured by loading towards either the plus end (b(i)) or the minus end (b(ii)). (c) Scheme to explain the dependence of the average unbinding force and the unbinding force distributions on loading rate and loading direction. A model where equilibrium is assumed to exist between the single-headed (1) and the double-headed (2) binding with the rate constants, k+ and k−, between two binding states. (d) Relation between the elastic modulus and unbinding force of kinesin molecules measured by the plus-end loading in the AMP–PNP state. Examples showing the force-extension relation obtained from the time course of bead displacement for the initial unbinding (dark green) and the subsequent unbinding (pink) that were observed during the movement of the kinesin-bound bead by manipulating with the laser trap along a microtubule at a constant rate. Short arrows show the moment at which the detachment of kinesin occurred. Long arrow shows the moment at which the transition to a steeper slope occurred. (Adapted from Kawaguchi et al. 2003.)