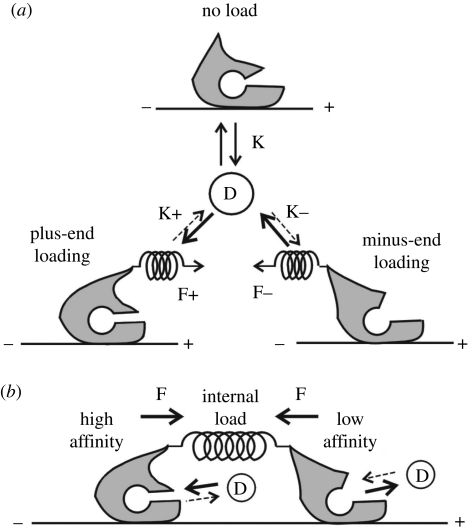
Figure 6.
Schematic illustrations showing equilibrium of ADP (D) binding to a kinesin–microtubule complex. (a) The effect of imposing load towards the plus end (F+) or the minus end (F−) of a microtubule on the binding equilibrium. The dissociation constants of ADP in a kinesin–microtubule complex in the absence of load (K), under plus-end loading, K+ and under minus-end loading, K−, are shown. (b) A possible role of an internal load within a two-headed kinesin molecule on the binding equilibrium. In (b), such a situation is illustrated that the internal load, which may be produced by extension of the link between the two heads upon binding to a microtubule, increases the binding affinity of ADP for the trailing head (left side) and decreases that for the leading head (right side). The internal load between the two heads is assumed to be equivalent to the plus-end loading for the trailing head and the minus-end loading for the leading head. (Adapted from Uemura & Ishiwata 2003.)