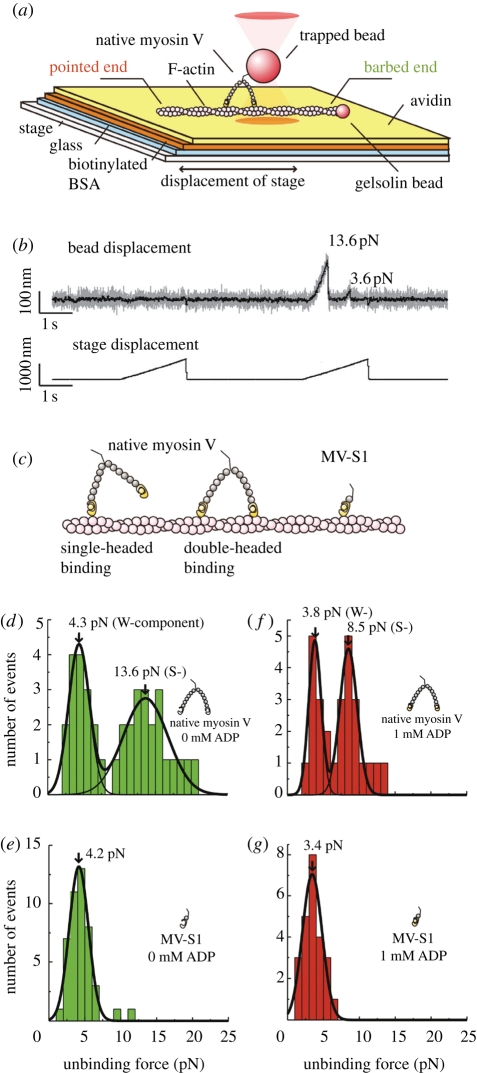
Figure 7.
Measurements probing the binding mode of myosin V. (a) Schematic illustration of the experimental system. A polystyrene bead (diameter 1 µm), to which a single myosin molecule (native myosin V or single-headed MV-S1) is attached, is held with optical tweezers and brought close to a 10 per cent-biotinylated actin filament, immobilized on a glass surface via the biotin–avidin interaction. The polarity of the actin filament was determined using a gelsolin-coated bead (diameter 0.2 µm), which specifically attaches to its barbed end. (b) A typical trace of bead displacement for native myosin V in the nucleotide-free state. Both the double- and the single-headed interactions were detected during the same pulling event. (c) Schematic representation of the binding modes of native myosin V and of the MV-S1 construct. Unbinding force distributions for native myosin V in the nucleotide-free (d) and the ADP-bound state (f) and for single-headed MV-S1 in the nucleotide-free (e) and the ADP-bound state (f). (Adapted from Mikhailenko et al. 2008.)