Abstract
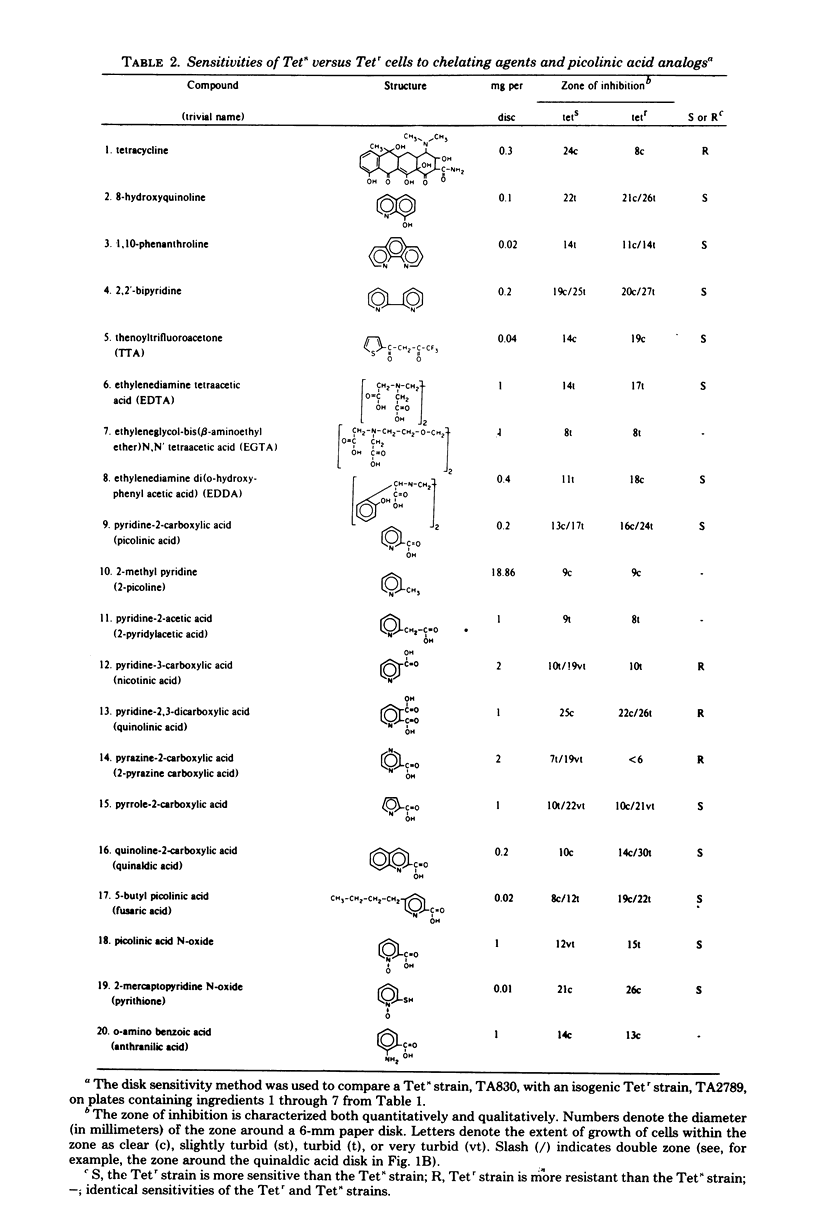
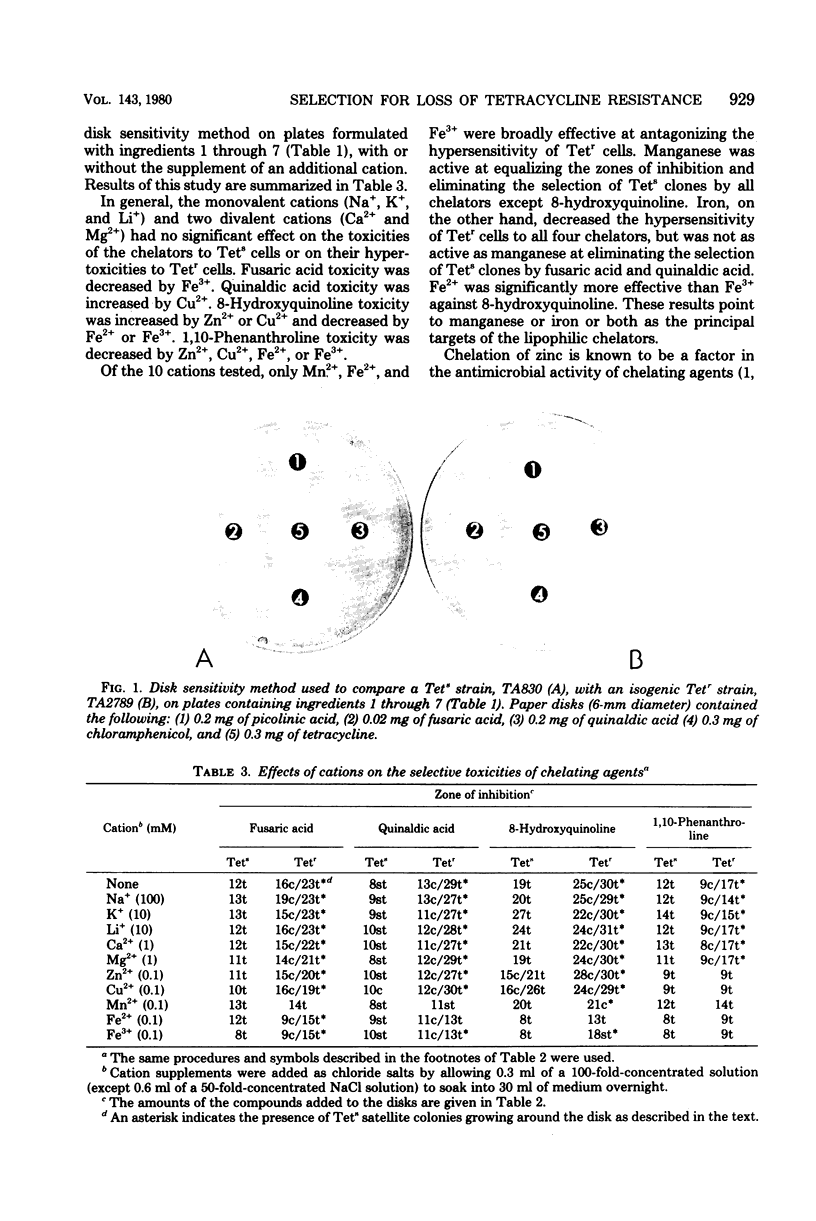
A simple technique has been devised that allows direct plate selection of tetracycline-sensitive clones from a predominantly tetracycline-resistant population. The technique is especially useful in genetic methodologies based on the use of tetracycline resistance transposons, such as Tn10. Potential uses of the method include selection of deletion mutants, fine-structure mapping, generalized mapping, construction of multiply marked strains, elimination of tetracycline resistance transposons and plasmids and cloning. The technique is based on our finding that tetracycline-resistant cells are hypersensitive to lipophilic chelating agents, such as fusaric acid. This finding supports the contention that certain metal ions critically facilitate tetracycline uptake and leads us to suggest possible molecular mechanisms for tetracycline resistance.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Calvo J. M., Goodman M., Salgo M., Capes N. Salmonella locus affecting phosphoenolpyruvate synthase activity identified by a deletion analysis. J Bacteriol. 1971 Apr;106(1):286–288. doi: 10.1128/jb.106.1.286-288.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Hutchison J. D. Selectivity of cation chelation to tetracyclines: evidence for special conformation of calcium chelate. Biochem Biophys Res Commun. 1971 May 7;43(3):625–630. doi: 10.1016/0006-291x(71)90660-7. [DOI] [PubMed] [Google Scholar]
- Chandler C. J., Segel I. H. Mechanism of the antimicrobial action of pyrithione: effects on membrane transport, ATP levels, and protein synthesis. Antimicrob Agents Chemother. 1978 Jul;14(1):60–68. doi: 10.1128/aac.14.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Howe T. G. Bacterial resistance to the tetracyclines. Microbiol Rev. 1978 Dec;42(4):707–724. doi: 10.1128/mr.42.4.707-724.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coibion C., Laszlo P. Binding of the alkali metal cations to tetracycline. Biochem Pharmacol. 1979 Apr 15;28(8):1367–1372. doi: 10.1016/0006-2952(79)90439-8. [DOI] [PubMed] [Google Scholar]
- Collins J. J., Alder C. R., Fernandez-Pol J. A., Court D., Johnson G. S. Transient growth inhibition of Escherichia coli K-12 by ion chelators: "in vivo" inhibition of ribonucleic acid synthesis. J Bacteriol. 1979 Jun;138(3):923–932. doi: 10.1128/jb.138.3.923-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN T. J., GODFREY A. RESISTANCE OF ESCHERICHIA COLI TO TETRACYCLINES. Biochem J. 1965 Jan;94:54–60. doi: 10.1042/bj0940054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Inhibition of aconitase by chelation of transition metals causing inhibition of sporulation in Bacillus subtilis. J Biol Chem. 1968 Oct 25;243(20):5289–5295. [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R. S., Creanor J. The mechanism of inhibition of ribonucleic acid synthesis by 8-hydroxyquinoline and the antibiotic lomofungin. Biochem J. 1975 Jun;147(3):401–410. doi: 10.1042/bj1470401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda R. C., Tanabe J. H., Knigge K. M., Markovitz A. Identification by deletion analysis of an inducible protein required for plasmid pSC101-mediated tetracycline resistance. Plasmid. 1979 Jul;2(3):417–425. doi: 10.1016/0147-619x(79)90025-8. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kazawa T., Mitsuhashi S. Antibacterial and inducer activities for tetracycline resistance by its derivates and analogues. Microbiol Immunol. 1977;21(2):59–67. doi: 10.1111/j.1348-0421.1977.tb02808.x. [DOI] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Adler C. R., Collins J. J., Court D. Role of the spoT gene product and manganese ion in the metabolism of guanosine 5'-diphosphate 3'-diphosphate in Escherichia coli. J Biol Chem. 1979 Jun 25;254(12):5483–5487. [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Reichardt K., Botstein D. Inversions and deletions of the Salmonella chromosome generated by the translocatable tetracycline resistance element Tn10. J Mol Biol. 1979 Jan 5;127(1):89–115. doi: 10.1016/0022-2836(79)90461-3. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Ross D. G. Translocation and other recombination events involving the tetracycline-resistance element Tn10. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1233–1246. doi: 10.1101/sqb.1979.043.01.140. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Plasmid-determined tetracycline resistance involves new transport systems for tetracycline. Nature. 1978 Nov 2;276(5683):90–92. doi: 10.1038/276090a0. [DOI] [PubMed] [Google Scholar]
- McMurry L., Levy S. B. Two transport systems for tetracycline in sensitive Escherichia coli: critical role for an initial rapid uptake system insensitive to energy inhibitors. Antimicrob Agents Chemother. 1978 Aug;14(2):201–209. doi: 10.1128/aac.14.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Ames G. F. Evidence for a common mechanism for the insertion of the Tn10 transposon and for the generation of Tn10-stimulated deletions. Mol Gen Genet. 1978 Oct 30;166(2):217–223. doi: 10.1007/BF00285924. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krausz J. Action of 12 tetracyclines on susceptible and resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1973 Sep;4(3):237–247. doi: 10.1128/aac.4.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z. Influence of magnesium and manganese on some biological and physical properties of tetracycline. J Bacteriol. 1972 May;110(2):468–476. doi: 10.1128/jb.110.2.468-476.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- WEINBERG E. D. The mutual effects of antimicrobial compounds and metallic cations. Bacteriol Rev. 1957 Mar;21(1):46–68. doi: 10.1128/br.21.1.46-68.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentmyer G. A. INHIBITION OF METAL CATALYSIS AS A FUNGISTATIC MECHANISM. Science. 1944 Sep 29;100(2596):294–295. doi: 10.1126/science.100.2596.294. [DOI] [PubMed] [Google Scholar]



