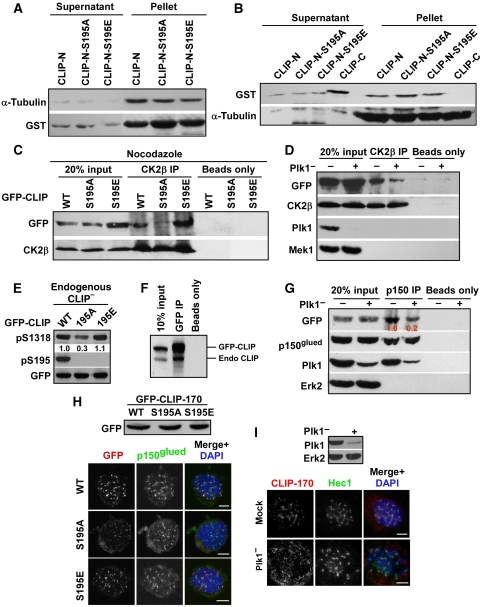
Figure 4.
Plk1 phosphorylation of CLIP-170-S195 is essential for its association with CK2. (A) After incubation with GST-CLIP-170-N-aa 1–364 (WT, S195A or S195E) proteins bound on glutathione-agarose beads, microtubules associated with the GST-fusion proteins were separated by low-speed centrifugation, followed by western blotting. (B) After purified GST-CLIP-170 proteins were incubated with microtubules, the supernatants and pellets were separated by ultracentrifugation. Microtubules and associated materials were analysed by western blot. CLIP-170-N: aa 1–364, CLIP-170-C: aa 1089–1392. (C) CLIP-170-depleted HeLa cells were transfected with rat GFP-CLIP-170, treated with nocodazole, and immunoprecipitated with CK2β antibodies, followed by western blotting. Protein A/G beads only serve as washing controls. (D) HeLa cells were depleted of Plk1, re-transfected with GFP-CLIP-170, treated with nocodazole, and immunoprecipitated with CK2β antibodies, followed by western blotting. Protein A/G beads only serve as washing controls. (E) CLIP-170-S195 phosphorylation affects S1318 phosphorylation. Cells as in (C) were immunoblotted. Numbers indicate the relative pS1318 intensity. (F) 293T cells were transfected with GFP-CLIP-170-S195A and harvested. Lysates were subjected to anti-GFP IP, followed by anti-CLIP-170 western blotting. Positions of GFP-CLIP-170 and endogenous CLIP-170 are indicated on the right. (G) Transfected cells as in (D) were immunoprecipitated with p150glued antibodies, followed by western blotting. Numbers indicate the relative GFP-CLIP-170 co-immunoprecipitated. (H) Kinetochore localization of CLIP-170 is S195-phosphorylation dependent. HeLa cells were co-transfected with pBS/U6-CLIP-170 and rat GFP-CLIP-170 (WT or S195 mutants) at a ratio of 2:1 and immunostained. Efficient protein expression of CLIP-170 constructs is indicated in the upper panel. (I) Kinetochore localization of CLIP-170 is Plk1 dependent. HeLa cells were depleted of Plk1, treated with nocodazole for 2 h, and co-stained with antibodies against CLIP-170 and Hec1. Bars, 5 μm.