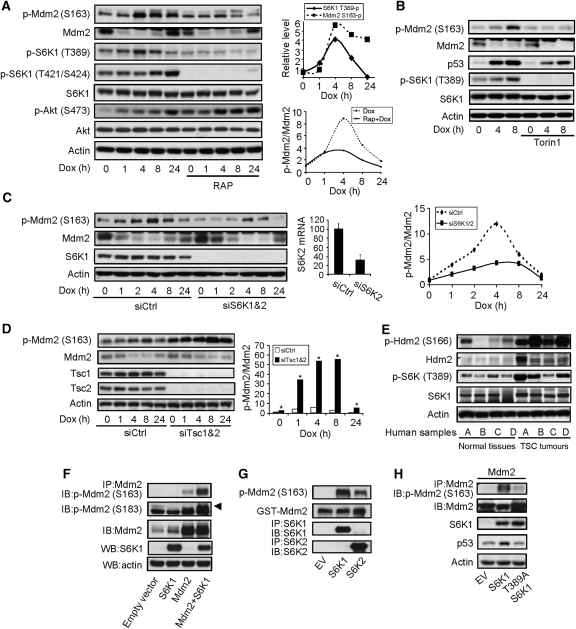
Figure 1.
Genotoxic stress induced Mdm2 S163 phosphorylation through mTOR-S6K. (A) Dox treatment led to Mdm2 S163 phosphorylation in primary MEFs, which was blocked by rapamycin pretreatment. MEFs were pretreated with or without 1 nM of rapamycin for 1 h before adding Dox to a final concentration of 1 μM for different periods of time. Phosphorylation and protein levels of Mdm2, S6K1, and Akt were analysed by western blot. (Right upper panel) Quantitation data of S6K1 T389 phosphorylation and Mdm2 S163 phosphorylation. (Right bottom panel) Quantitation data of Mdm2 S163 phosphorylation normalized to Mdm2 protein levels. The value of p-Mdm2 S163 at time 0 in the absence of RAP was set at 1.0. (B) Dox-induced Mdm2 S163 phosphorylation was blocked by Torin1. The experiments were carried out as in Figure 1A except that 250 nM of Torin1 was used to replace Rapamycin. The value of p-Mdm2 S163 at time 0 in the absence of Torin1 was set at 1.0. (C) Knockdown of S6K1 and 2 led to hypophosphorylation of Mdm2. S6K1 and S6K2 were knocked down with siRNA in primary MEFs for 48 h before addition of Dox. Middle panel shows the mRNA levels of S6K2 after knockdown (because of the weak activity of S6K2 antibodies). (Right panel) Quantitation data of Mdm2 S163 phosphorylation normalized to Mdm2 protein levels. The value of p-Mdm2 S163 at time 0 in the presence of control siRNA was set at 1.0. (D) Knockdown of Tsc1 and Tsc2 led to enhanced Mdm2 S163 phosphorylation in MEFs. Tsc1 and Tsc2 were knocked down by siRNA in MEFs for 48 h before addition of Dox. The phosphorylation of Mdm2 S163, Mdm2, and Tsc1/2 were analysed by western blot. (Right panel) Quantitation data of Mdm2 S163 phosphorylation normalized to Mdm2 protein levels. The value of p-Mdm2 S163 at time 0 in the presence of control siRNA was set at 1.0. (E) Tumour kidney tissues from four TSC patients (A–D) showed enhanced Hdm2 S166 phosphorylation. TSC tumour samples and matched normal tissues (four each) were homogenized and the phosphorylation of Hdm2 S166 and S6K1 T389 was analysed by western blot. (F) Co-expression of S6K1 led to Mdm2 phosphorylation on S163. Empty vector or S6K1 was co-transfected with Mdm2 in 293T cells, which were treated with Dox for 4 h. Mdm2 was immunoprecipitated and phosphorylation at S163 or S183 (upper arrow) was analysed by western blot. (G) In vitro kinase assay showed that S6K1 could directly phosphorylate Mdm2. HA-tagged S6K1 or HA-tagged S6K2 was expressed in 293T cells and was immunoprecipitated from the cells. GST-Mdm2, which was expressed and purified from bacteria, was used as substrate to incubate with immunoprecipitated S6K1 or S6K2. Phosphorylation of Mdm2 S163 was determined by western blot. (H) Requirement for S6K1 T389 for Mdm2 S163 phosphorylation. S6K1 or S6K1 T389A mutant was expressed together with Mdm2 in 293T cells, and then Mdm2 was immunoprecipitated and its S163 phosphorylation was determined by western blot.