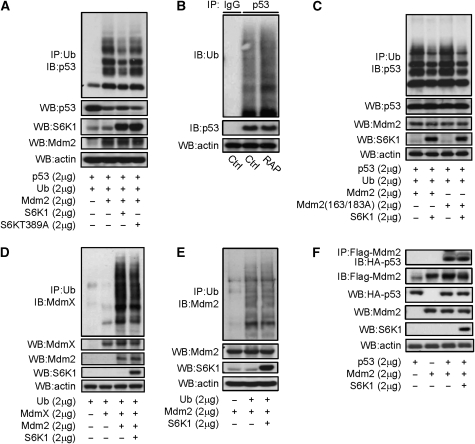
Figure 5.
S6K is a negative regulator of Mdm2-mediated p53 ubiquitination. (A) S6K1, but not T389A mutant S6K1, inhibited Mdm2-mediated p53 ubiquitination in H1299 cells. A measure of 2 μg of Flag-p53, HA-ubiquitin, and Mdm2, together with S6K1 or mutant S6KT389A, were expressed in H1299 cells, which were then treated with 10 μM of MG132 for 4 h before being harvested. Ubiquitinated proteins were immunoprecipitated with anti-HA (Ub) antibodies. Ubiquitinated p53 was detected by western blot using anti-p53 antibodies. (B) Inhibition of mTOR-S6K by rapamycin enhanced endogenous p53 ubiquitination in MEFs. MEFs were treated with or without rapamycin for 4 h, then followed by 10 μM of MG132 for 4 h before being harvested. Endogenous p53 was immunoprecipitated with antibodies against p53. Ubiquitinated p53 was detected with anti-ubiquitin antibodies. (C) S163/183A mutant Mdm2 could mediate p53 ubiquitination, which was inhibited by co-expression of S6K1 in H1299 cells. (D) S6K1 expression modestly inhibited Mdm2-mediated MdmX ubiquitination in H1299 cells. A measure of 2 μg each of HA-ubiquitin, MdmX and Mdm2, together with S6K1 were expressed in H1299 cells, which were then treated with 10 μM of MG132 for 4 h before being harvested. Ubiquitinated proteins were immunoprecipitated with anti-HA antibodies. Ubiquitinated MdmX was detected by western blot using anti-MdmX antibodies. (E) S6K1 expression showed no effect on Mdm2 self-ubiquitination in H1299 cells. A measure of 2 μg each of HA-ubiquitin and Mdm2, together with S6K1 were expressed in H1299 cells, which were then treated with 10 μM of MG132 for 4 h before being harvested. Ubiquitinated proteins were immunoprecipitated with anti-HA antibodies. Ubiquitinated Mdm2 was detected by western blot using anti-Mdm2 antibodies. (F) S6K1 did not show much effect on Mdm2–p53 interaction. Flag-Mdm2 and HA-p53 were expressed together with or without S6K1 in 293T cells, and Flag-Mdm2 was then immunoprecipitated and the associated p53 was determined by western blot.