Figure 1.
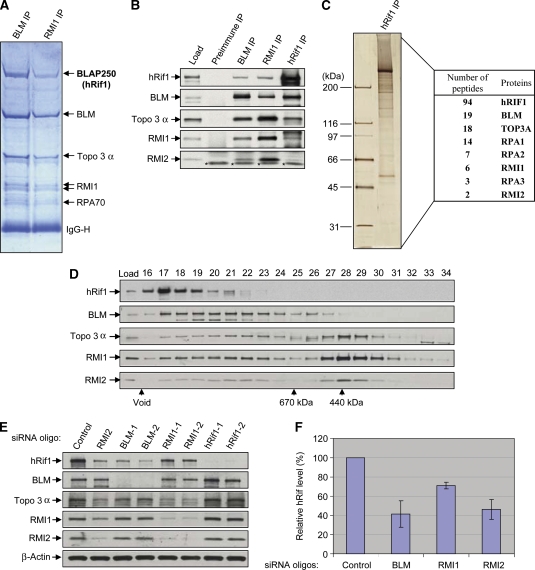
hRif1 is a new component of BLM complex and its stability depends on the other components of the complex. (A) A Coomassie blue-stained SDS gel showing that hRif1 (previously named BLAP250) is present in complexes immunoprecipitated by both BLM and RMI1 antibodies. The major polypeptides on the gel (marked with arrows) were indentified by mass spectrometry. This figure is reproduced from Figure 1 of a previous publication (Yin et al, 2005) for readers' convenience. (B) Immunoblotting shows that hRif1 coimmunoprecipitates with other components of the BLM complex. Preimmune serum was used as a negative control. The nuclear extract input is shown as load. A cross-reactive polypeptide is indicated with asterisks. (C) A silver-stained SDS gel showing the polypeptides immunopurified by an hRif1 antibody from peak fractions of Rif1 after fractionation of HeLa nuclear extract by Superose 6 column (see D). The proteins identified by mass spectrometry and the number of peptides discovered for each protein are listed in the table on the right. (D) Immunoblotting shows that the Superose 6 fractionation profile of hRif1 overlaps with those of the BLM complex components. The fractions corresponding to molecular weight standards are indicated at the bottom. The profiles of the BLM complex components (the bottom four panels) are reproduced from Supplementary Figure S1 of a previous publication (Xu et al, 2008) for comparison. (E, F) Immunoblotting (E) and its quantification (F) show that the hRif1 protein levels are reduced in HeLa cells depleted of BLM, RMI1, or RMI2 by siRNA. Immunoblotting of β-actin was used as a loading control. (F) The relative hRif1 levels were shown, with that of control siRNA-treated cells being set as 100%. Quantification was done using data from at least four independent experiments for each protein. The data represent the mean values, and the error bars represent standard errors.