Abstract
There must be something unique about a class of drugs (discovered and developed in the mid-1940s) where there are more than 130 ongoing clinical trials currently listed. Tetracyclines were developed as a result of the screening of soil samples for antibiotic organisms. The first of these compounds chlortetracycline was introduced in 1948. Soon after their development tetracyclines were found to be highly effective against various pathogens including rickettsiae, Gram-positive, and Gram-negative bacteria, thus, becoming a class of broad-spectrum antibiotics. The mechanism of action of tetracyclines is thought to be related to the inhibition of protein synthesis by binding to the 30S bacterial ribosome. Tetracyclines are also an effective anti-malarial drug. Over time, many other “protective” actions have been described for tetracyclines. Minocycline, which can readily cross cell membranes, is known to be a potent anti-apoptotic agent. Its mechanism of action appears to relate to specific effects exerted on apoptosis signaling pathways. Another tetracycline, doxycycline is known to exert antiprotease activities. Doxycycline can inhibit matrix metalloproteinases, which contribute to tissue destruction activities in diseases such as gingivitis. A large body of literature has provided additional evidence for the “beneficial” actions of tetracyclines, including their ability to act as oxygen radical scavengers and anti-inflammatory agents. This increasing volume of published work and ongoing clinical trials supports the notion that a more systematic examination of their possible therapeutic uses is warranted. This review provides a summary of tetracycline's multiple mechanisms of action and while using the effects on the heart as an example, this review also notes their potential to benefit patients suffering from various pathologies such as cancer, Rosacea, and Parkinson's disease.
Keywords: protease inhibitors, doxycycline, minocycline
Classical Uses of Tetracyclines
the tetracyclines are an aging family of broad-spectrum antibiotics. The parent compound chlortetracycline (tradename Aureomycin) was first isolated from Streptomyces aureofaciens in 1947 (21). Soon after, other natural tetracyclines were isolated, including tetracycline, for which the family of molecules is named. Since then, the modifications of naturally occurring tetracyclines and the synthesis of novel compounds within the tetracycline family have generated many compounds. Two of the more common semisynthetic tetracyclines used clinically as antibiotics are doxycycline (DOX) and minocycline (MIN), which are essentially well-tolerated and safe compounds. Because of its broad-spectrum antibiotic efficacy, DOX is indicated for the treatment of a variety of infections, including anthrax, chlamydial infections, community-acquired pneumonia, Lyme disease, cholera, syphilis, Yersinia pestis (plague), periodontal infections, and others. MIN also displays broad-spectrum efficacy and is most often used clinically in the treatment of severe acne, but it is also indicated for many of the same infections as DOX (50).
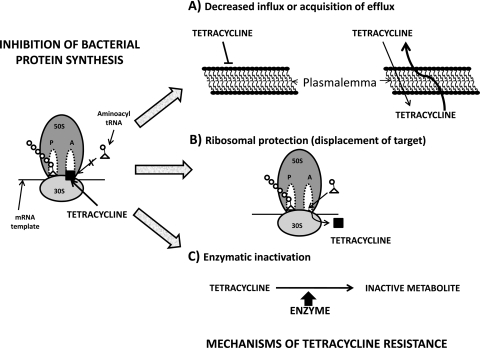
The tetracyclines exert their antibiotic effect primarily by binding to the bacterial ribosome and halting protein synthesis (39). Bacterial ribosomes have a high-affinity binding site located on the 30S subunit and multiple low-affinity sites on both the 30S and 50S subunits (89). Upon binding the ribosome, the tetracyclines allosterically inhibit binding of the amino acyl-tRNA at the acceptor site (A-site), and protein synthesis ceases (76). However, the use of tetracyclines has declined in recent decades due to the emergence of resistant strains of bacteria (Fig. 1). The primary mechanism of resistance is mediated by increased drug efflux out of the cell by a family of Tet proteins located on the cytoplasmic surface of the cell membrane (55, 97). Whereas an understanding of the mechanisms of bacterial resistance (42) to the tetracyclines is important clinically, it also has led to the development of the Tet regulatory system, an important transcriptional regulation tool that is used extensively for targeted gene regulation in eukaryotes (35).
Fig. 1.
Illustration of the means by which tetracyclines may lose their protein synthesis inhibitory capacity in pathogens such as bacteria. This may occur secondary to the enhanced extrusion of the drug, decreased entry, displacement from the target (ribosomes), or enzymatic inactivation.
Tetracyclines are also effective but slow-acting anti-malarial drugs. DOX-treated parasites appear morphologically normal until late in the second cycle of treatment but fail to develop into merozoites (15). DOX specifically impairs the expression of apicoplast genes. Apicoplast (nonphotosynthetic major organelles found in cells of plants) are abnormal in the progeny of DOX-treated parasites. The loss of apicoplast function in the progeny of treated parasites leads to a slow but potent anti-malarial effect.
Chemical Properties
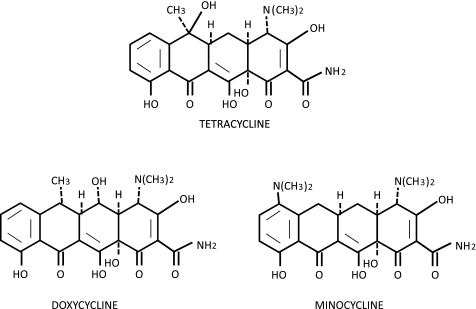
While the complex chemistry of the tetracyclines is beyond the scope of this review, a basic understanding of the important features of these compounds is necessary to appreciate their ability to interact with such a wide variety of biomolecules. Tetracycline, DOX, and MIN are all composed of a four-ring core to which are attached various side groups (Fig. 2). The dimethylamino group at the C4 carbon on the upper half of the molecule has been shown to be necessary for antimicrobial activity. 4-De-dimethylamino tetracyclines, also called chemically modified tetracyclines (CMTs), lack antimicrobial activity in vivo presumably due to the inability of the molecule to adapt a zwitterionic form necessary for activity (56). However, CMTs do retain the ability to bind other nonmicrobial targets, such as matrix metalloproteinases (MMPs), facilitating their use in the treatment of other disease processes (32). The oxygen-rich lower half of the molecule is critical for binding to both prokaryotic and eukaryotic targets, and interference with this region reduces or eliminates the effectiveness of the drug (33). This region is relevant as site for metal ion chelation. Binding of tetracyclines to proteins, including TetR, may be greatly enhanced when the tetracycline is complexed with divalent metal ions such as Ca2+ or Mg2+ (86). The binding of tetracyclines to MMPs is thought to be mediated by the chelation of structural and catalytic Zn2+ ions within the enzyme (Fig. 3) (33, 70). In addition, binding to the bacterial ribosome involves binding to RNA-bound Mg2+ (29). The strength of tetracycline-metal interaction is dependent on both the tetracycline and the metal ion present. In general, the affinity of the tetracyclines for different divalent metals is in order of decreasing affinity: Cu2+ > Co2+ = Fe2+ > Zn2+ > Mn2+ > Mg2+ > Ca2+ (61). The relative affinities of different tetracyclines for a given metal also differ and are highly dependent on pH and the presence of other metal ions (4, 6, 53). The relative superiority of DOX as an MMP inhibitor is due to its increased affinity for Zn2+ compared with tetracycline or MIN (7). In general, there is a direct relationship between lipophilicity and activity against Gram-positive bacteria. The lipophilicity of tetracycline, DOX, and MIN, as determined by partitioning between octanol and aqueous buffer, has been determined to be 0.025, 0.600, and 1.1, respectively (11), and the minimum inhibitory concentration against Staphylococcus aureus is 0.21, 0.19, and 0.10 μg/ml, respectively (5). Lipophilicity also affects tissue distribution. MIN is able to cross the blood-brain barrier much more readily than DOX or tetracycline. MIN attains levels in the brain nearly threefold higher than DOX, and tetracycline is undetectable in the brain (3).
Fig. 2.
Chemical structure of tetracyclines.
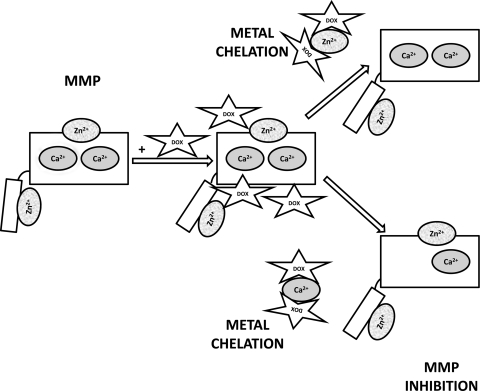
Fig. 3.
Illustration of the means by which doxycycline through zinc and calcium chelation may act to inhibit matrix metalloproteinases (MMPs). Both ions are crucial to allow for enzyme activity.
Matrix Metalloproteinase Inhibition by Tetracyclines
Probably the best characterized non-antimicrobial property of the tetracyclines is their ability to inhibit members of the MMP family of endopeptidases. The MMPs are a family of zinc-dependent proteases that are involved in many physiological and pathophysiological processes including embryogenesis, tissue remodeling, inflammation, and tumor invasion (58). MMPs can be subdivided based on crude substrate specificities into the collagenases, gelatinases, stromelysins, and membrane-type MMPs (MT-MMPs) (82). The collagenase group includes MMP-1, MMP-8, and MMP-13, and all cleave fibrillar collagens (types I and III). Collagen fragments subsequently denature into gelatins. The gelatinases, which include MMP-2 and MMP-9, proteolyze the gelatins. The gelatinases also degrade basement membrane collagen (type IV). The stromelysins includes MMP-3, MMP-7, MMP-10, and MMP-11, and they are capable of degrading proteoglycans, laminin, fibronectin, collagen IV, and others. The cell membrane-anchored MT-MMP include six different MMP, of which MT1-MMP is the best characterized (19).
Inhibition of MMPs is beneficial in many pathological conditions in which MMP-mediated proteolysis of the extracellular matrix (ECM) contributes to the pathogenesis, such as heart remodeling, tumor invasion, and inflammation (58, 64, 67). Numerous synthetic compounds with potent and specific in vitro MMP inhibitory activities were developed, and many have entered clinical trials (64). However, because of the lack of efficacy and serious musculoskeletal side effects, none progressed to phase III clinical trials. Currently, the only clinically available MMP inhibitor is DOX, which is marketed as Periostat, and it is indicated only for the treatment of periodontitis (64). Following the seminal experiment by Golub et al. (31) showing that MIN inhibited collagenase derived from rat gingiva, tetracyclines became increasingly studied for their ability to inhibit MMPs.
The mechanism by which tetracyclines inhibit MMPs has not been completely elucidated. It is believed that they exert their anti-proteolytic effects by both direct inhibition of MMPs and by inhibiting their expression. Direct inhibition of MMPs appears to be mediated by an interaction between the tetracycline molecule and metal ions within the MMP. MMP inhibition can be reversed by the addition of millimolar amounts of Ca2+ (30) or micromolar amounts of Zn2+ (100). Interestingly, it appears that the mechanism of inhibition is dependent on chelation of structural metals rather than chelation of the active site Zn2+ (78). In addition, it was recently shown that DOX binds to MMP-7 near the structural Zn2+ and Ca2+ ions and does not bind to the catalytic Zn2+ (28) (Fig. 3). The effectiveness of tetracycline inhibition against various MMPs depends on the tetracycline species, MMP species, and the pH. It has been shown that DOX is more potent than MIN or tetracycline against collagenases purified from rabbit corneas, with IC50 values of 15 μM, 190 μM, and 350 μM, respectively, and this trend may be explained by the relatively high affinity of DOX and low affinity of tetracycline for Zn2+ (7). The IC50 values for DOX against the collagenases MMP-8, MMP-13, and MMP-1 is 1–10 μM, 5–30 μM, and >200 μM, respectively (36, 34, 79); the reasons for the differences in inhibition of the various MMPs are not clear. The pH of the system also affects inhibition as evidenced by the ability of DOX to inhibit MMP-8 at pH > 7.1 and inability to inhibit at pH < 7.1 (77). In addition to inhibiting MMPs directly, tetracyclines also inhibit MMP synthesis. DOX inhibited cytokine-induced MMP-8 mRNA and protein accumulation in cultured rat synovial fibroblasts (41). In cultured human skin fibroblasts, tetracycline inhibited interleukin-1 (IL-1)-induced MMP-3 expression (48). Since MMP transcription is induced by a host of proinflammatory cytokines and other growth factors, including IL-1, IL-6, tumor necrosis factor-α (TNF-α), epidermal growth factor, and others (13), it is likely that these upstream signaling cascades leading to MMP expression are important targets of tetracyclines. An interesting consequence of MMP inhibition is the indirect inhibition of serine proteases. MMPs can inactivate serine protease inhibitors (SERPINs) (17, 57), and MMP inhibition with DOX or CMTs preserves SERPINs thereby blocking serine protease activity (14, 30, 37, 80, 81).
MMP activity is upregulated early following a myocardial infarction (MI), suggesting an important role in mediating the acute injury and healing processes of the heart, including inflammation, angiogenesis, scar formation, and remodeling. However, early MMP activity may be a double-edged sword, leading also to early damage of the cardiac ECM. MMP activity increases within minutes of ischemia, reflecting activation of preformed latent MMPs within the myocardium (16, 23, 68). The simultaneous appearance of type I collagen fragments in serum indicates that this early MMP activity contributes to significant collagen proteolysis (90). This early damage may set the stage for long-term adverse remodeling. In addition to their role in mediating post-MI healing and remodeling, there is emerging evidence that the MMPs may actually contribute to myocyte death and dysfunction following ischemia-reperfusion (I-R) injury. MMP-2 activity is increased in isolated, perfused rat hearts following I-R, and treatment with DOX attenuated the increase in MMP-2 activity upon reperfusion and improved the recovery of contractile function (10). MMP-2 activation following I-R resulted also in proteolysis of troponin I and myosin light chain, and DOX prevented their proteolysis (74, 94). It is possible that MMP-mediated disruption of the normal myocyte-matrix architecture leads to myocyte cell death, since disruption of normal cell-ECM interactions has been shown to contribute to the induction of myocyte apoptosis both in vitro (71) and in vivo (18). These studies demonstrate that inhibition of MMP activity holds promise for salvaging myocytes and preserving cardiac structure and function post-MI. However, for pharmacological MMP inhibition to be beneficial, the timing of MMP inhibition will likely be important. For example, early short-term MMP inhibition post-MI (<48 h) may block the early damage to the ECM while still allowing later events (>48 h), such as inflammation and wound healing, to proceed normally. Indeed, significant anti-remodeling (i.e., beneficial) effects can be observed in the infarcted heart when DOX is given for a limited period of time after injury (27).
Reactive Oxygen Species Scavenging by Tetracyclines
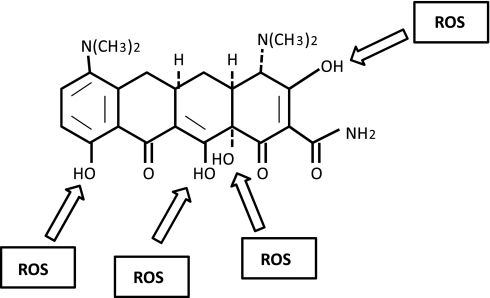
Another well-characterized nonantimicrobial property of the tetracyclines is their ability to scavenge reactive oxygen species (ROS). Excess ROS are produced under many pathological conditions, including myocardial I-R injury and inflammatory processes, and can lead to the oxidative destruction or dysfunction of many cellular constituents. ROS are highly reactive and include the free radicals superoxide (O2·−) and hydroxyl radical (OH•), the non-free radicals hydrogen peroxide (H2O2), hypochloride (HOCl), and peroxynitrite (ONOO−) (62). DOX, MIN, and tetracycline all have a multiple-substituted phenol ring, similar to vitamin E. The phenol ring is key to the ROS-scavenging abilities of these compounds. The reaction of the phenol ring with a free radical generates a phenolic radical that becomes relatively stable and unreactive due to resonance stabilization and steric hindrance by the phenol ring side groups (52). MIN directly scavenge ROS in several cell-free mixed-radical assays with a potency comparable to vitamin E, and its scavenging ability was not dependent on chelation of Fe2+ (i.e., inhibition of the Fenton reaction) (52). Depending on the assay used, MIN had an IC50 of 3–40 μM and is 9–250 times more potent as scavenger than that of DOX and 200–300 times more potent than of tetracycline. The superior scavenging ability of MIN is likely due to the presence of the diethyamino group on the phenolic carbon (Fig. 4), which is unique to MIN and provides improved steric hindrance (52). Other groups have shown DOX to be an effective ROS scavenger. DOX inhibits both HOCl and a mixture of neutrophil-derived ROS in vitro, with an IC50 of ∼2 μM (43). In addition, DOX inhibits HOCl-mediated activation of MMP-1 from osteosarcoma cells, with an IC50 of ∼50 μM (66).
Fig. 4.
Illustration highlights minocycline hydroxyl radicals that may react with reactive oxygen species (ROC) thus yielding antioxidant activity.
The generation of ROS upon reperfusion of ischemic myocardium is thought to be one of the primary mediators of reperfusion injury. It has also been shown that administration of exogenous SOD (9, 93, 99) and/or catalase reduces the extent of I-R injury in canine (1, 47) and porcine (60) models. The addition of ONOO− (which degrades to NO2 and OH•) to isolated adult cardiac myocytes caused impaired calcium homeostasis and contractile dysfunction (54). DOX treatment normalized calcium transients and reduced the decline in contraction induced by ONOO−.
Anti-Apoptotic Effects of Tetracyclines
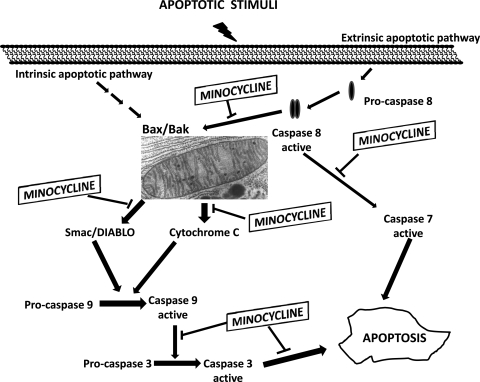
A key event in the execution of the apoptotic cascade is the activation of caspases, a family of cysteine proteases. Caspases can be divided into upstream “initiator” caspases, which function to activate downstream caspases, and the downstream “executioner” caspases that are responsible for the proteolytic destruction of the cell (25). Caspases may be activated by either the extrinsic (death receptor) pathway or the intrinsic (mitochondrial) pathway. In the extrinsic pathway (88), death signal proteins such as TNF-α and Fas ligand bind to their cell surface receptors and, through signal transduction cascades, lead to the activation of an initiator caspase caspase-8. Caspase-8 in turn activates downstream caspases, such as caspase-3. In the intrinsic pathway (72), a variety of extracellular and intracellular stress stimuli converge on the mitochondria to destabilize it and cause the release of pro-apoptotic factors into the cytosol. A key factor mediating mitochondrial destabilization is opening of the mitochondrial permeability transition pore (mPTP), a large, high-conductance pore that spans both the inner and outer mitochondrial membranes (40). Once the mPTP opens, there is an influx of water, which leads to swelling of the mitochondrial matrix and eventually rupture of the outer mitochondrial membrane. Cytochrome c and other pro-apoptotic proteins, such as Smac/DIABLO [mitochondria-derived activator of caspases/direct inhibitor of apoptosis protein (IAP) binding protein with low PI]and apoptosis-inducing factor (AIF) are released from the intermembrane space into the cytosol. Cytochrome c binds the cytosolic adapter protein Apaf-1, which allows the recruitment and activation of an initiator caspase caspase-9, which then activates downstream caspases (46). Smac/DIABLO binds and inhibits caspase inhibitor proteins thereby disinhibiting caspase activity (59). AIF translocates to the nucleus and induces chromatin condensation and DNA fragmentation in a caspase-independent manner (85). The mitochondrial apoptosis pathway is also partly regulated by the Bcl-2 family of proteins (12). Bcl-2 family members may be pro-apoptotic (Bax, Bad, Bid) or anti-apoptotic (Bcl-2, Bcl-xL).
There is an increasing body of evidence suggesting that the tetracyclines possess anti-apoptotic properties (Fig. 5). In a seminal study by Yrjanheikki et al. (101), it was found that MIN and DOX increased the survival of hippocampal neurons following global brain ischemia in gerbils, and this protection was associated with reduced caspase-1 expression. MIN was subsequently evaluated in several other models of neuronal injury and found to also be protective against Huntington's disease (8), traumatic brain injury (73), and Parkinson's disease (20). In each of these cases, the neuroprotection by MIN was associated with a reduction in caspase-1 and/or caspase-3 expression, suggesting MIN was protective by inhibiting the expression of key factors within the apoptotic cascade. In addition to inhibiting caspase expression, MIN has also been shown to inhibit caspase activity by blocking activation. Zhu et al. (103) demonstrated that MIN inhibits cytochrome c release and caspase-3 activation in mice with amyotrophic lateral sclerosis. Using isolated mitochondria, Zhu and colleagues also showed that MIN inhibited Ca2+, and Bid induced mitochondrial swelling and cytochrome c release, indicating that the mitochondria, and perhaps the mPTP, were direct targets of MIN (103).In a follow-up study, the same group showed that MIN inhibited mitochondrial release of cytochrome c, Smac/DIABLO, and AIF in both a culture model and an in vivo mouse model of Huntington's disease, indicating MIN inhibits both caspase-dependent (cytochrome c and Smac/DIABLO) and caspase-independent AIF mitochondrial death pathways (95). MIN also protected cultured renal epithelial cells from I-R injury and inhibited mitochondrial cytochrome c release (92). Interestingly, this group found that protection by MIN was dependent on MIN-induced upregulation of Bcl-2, as antisense-mediated downregulation of Bcl-2 abolished the protective effects of MIN. Taken together, these studies demonstrate that MIN, and possibly also DOX, possess anti-apoptotic effects mediated by inhibition of caspase expression and by mitochondrial stabilization.
Fig. 5.
Summarization of possible sites of action of minocycline on suppressing apoptotic signals in cells.
MIN has been shown to also improve myocyte survival and reduce infarct size, and this was correlated with reduced caspase expression and reduced mitochondrial release of cytochrome c and Smac/DIABLO (75). These studies demonstrate that blocking apoptosis by directly inhibiting factors within the apoptotic cascade can salvage myocytes and reduce infarct size. MIN has been shown to effectively block apoptosis in models of neuronal injury, and there is at least preliminary evidence that MIN can reduce infarct size by blocking apoptosis in the heart. Additional studies are needed to clarify this potentially important role of tetracyclines in the heart.
Anti-Inflammatory Effects of Tetracyclines
A class of compounds that can act as anti-proteolytic and ROS scavenger is likely to exert anti-inflammatory actions. It is well accepted in the dermatology field that rosacea is a disease of inflammatory nature (51). DOX are successfully used in the treatment of skin conditions such as acne and rosacea. Biopsies of inflammatory lesions of patients with acne yield increases in proinflammatory cytokines TNF-α and IL-1β (63). These cytokines are known inducers of increases in MMP levels and of their activity. ROS and nitric oxide (NO) have also been described as playing a role in the pathophysiology of rosacea (49). NO likely mediates increases in vessel permeability and edema and may support erythema development that occurs with rosacea. The beneficial effects of TTCs are likely associated with their aggregate “beneficial” actions including inhibiting proinflammatory cytokine levels, MMPs, and ROS. However, other actions add to their anti-inflammatory profile. MIN and to a lesser extent DOX can inhibit both pancreatic and nonpancreatic phospolipase A2 (65). This enzyme plays important roles in joint inflammation with a less clear participation in rosacea. The migration of white blood cells is critical for inflammation to occur. TTCs can inhibit neutrophil migration (22), adherence (26), and the proliferation of lymphocytes (87). Granuloma formation is a salient feature of many inflammatory diseases including rosacea. TTCs have been shown to inhibit granuloma formation of blood mononuclear cells in in vitro systems (996). Thus the cumulus of these features aggregates into the anti-inflammatory profile of TTCs.
Tetracycline Uptake by Tissues and Cells
Tetracyclines have been reported to concentrate at the site of tissue injury. In the 1970s investigators using radiolabeled tetracycline noted its capacity to accumulate in damaged myocardium and serve to diagnose infarcts (44, 45). Results demonstrated a correlation between infarct size, as determined by radiolabeled tetracycline, and serum creatine kinase. The ability of tetracycline to concentrate in other tissues is well known (2). Dentists take advantage of the high concentration of DOX (Periostat) in saliva as a means to treat gingivitis. To investigate tetracycline accumulation in gingival tissue, Yang et al. (98) examined the capacity of gingival fibroblasts to uptake the compounds. Gingival fibroblasts transport MIN in a concentration- and temperature-dependent manner. At steady state, the cellular/extracellular concentration ratio was >60 for MIN. The uptake of tetracyclines also has been observed in neutrophils and may partly explain high levels observed in injured tissues (91). Romero-Perez et al. (69) explored the capacity of MIN to accumulate in myocardial tissue and cells. MIN accumulated in myocardium severalfold greater than plasma levels. Accumulation was more pronounced in ischemic than normal myocardium. Cardiac fibroblasts and myocytes possess a comparable uptake system to that reported for gingival cells (98). Their intracellular concentration could in theory reach millimolar levels. At these concentrations mass action effects are likely seen where the drugs may at the same time exert potent antioxidant, anti-MMP, and other effects that in balance ultimately translate into cytoprotective effects.
Other Potential Uses of Tetracyclines
The pleiotropic properties of tetracyclines are not necessarily limited to those described above. Indeed, this class of drugs have been reported as exerting unique effects on complex pathologies. In an experimental simian immunodeficiency virus (SIV) model of HIV central nervous system (CNS) disease, MIN reduced the severity of encephalitis, suppressed viral load in the brain, and decreased the expression of CNS inflammatory markers (102). Tetracyclines also demonstrate protective effects on prion [PrP(Sc)]-mediated brain damage. In one study Syrian hamsters were injected intracerebrally with scrapie-infected brain homogenate that was coincubated with 1 mM tetracycline before inoculation (25). Animals showed a significant delay in the onset of clinical signs of disease and prolonged survival time. Effects were paralleled by a delay in the appearance of magnetic-resonance abnormalities in the thalamus, neuropathological changes, and PrP(Sc) accumulation. When tetracycline was preincubated with highly diluted scrapie-infected inoculum, one third of hamsters did not develop disease. Thus tetracyclines appear to reduce prion infectivity through a direct interaction with PrP(Sc) and are potentially useful for inactivation of bovine spongiform encephalopathy (BSE)- or variant Creutzfeldt-Jacob disease-contaminated products and prevention strategies.
There are few agents as acutely damaging to tissues and living organisms as mustard gas. This alkylating agent causes massive blistering of the skin and severely damages the lungs by activating proteases (including elastases and MMPs) among other effects. In a study by Guingabert et al. (38) guinea pigs were given mustard gas intratracheally (38). A group of animals were pretreated with DOX resulting in decreased gelatinase activity, decreased inflammation, and notable decrease in histological lung epithelial lesions. Acute respiratory distress syndrome (ARDS) develops in the setting of diseases such as sepsis. With ARDS an infiltration of the lungs by neutrophils can lead to a massive activation of the cells yielding local tissue destruction and possibly the death of the subject. The destruction of lung tissue can be documented in bronchial lavage by the presence of protease such as elastases, MMP, collagen, and elastin fragments. In a study by Steinberg et al. (83) the prophylactic use of the CMT COL-3 was examined in a chronic insidious onset animal model of sepsis-induced ARDS. COL-3 prevented the development of ARDS and also prevented septic shock. The emergence of new epidemics where ARDS may be an important component to the cause of severe disease or death raises the spectrum of the possible use of tetracyclines to prevent or limit the development of respiratory system complications. Such is the case for H1N1 influenza and SARS (severe acute respiratory distress syndrome caused by a coronavirus). A potential scenario may be where there is high risk of exposure and contamination by a mutated version of a virus (such as in the case of the H1N1 influenza) by medical personnel where the use of tetracyclines may be justified as a means of preventing serious complications from developing upon exposure and infection.
CMT (in particular COL-3) have also been examined for their anti-cancer therapeutic potential. MMP are involved in tumor metastasis and angiogenesis and are overexpressed in Kaposi's sarcoma (KS) cells. In a phase II study, there were significant declines in MMP-2 and MMP-9 plasma levels from baseline to minimum value with treatment, and the most common adverse events were photosensitivity and rash. COL-3, when administered at 50 mg/day, demonstrated anti-tumor activity similar to other promising investigational KS drugs.
Ongoing Clinical Trials
Table 1 summarizes the most relevant clinical trials implemented (excluding those associated with their antibiotic or dermatologic effects). As can be ascertained from the list, this class of drugs is receiving a great amount of attention pertaining to their apparent capacity to beneficially modulate various apparently unrelated diseases. The table also includes (in very general terms) the apparent cause associated with a particular disease where tetracyclines are being tested.
Table 1.
Most relevant clinical trials implemented using minocycline, doxycycline, and chemically modified tetracycline COL-3
| Disease | Cause |
|---|---|
| Minocycline | |
| Acute kidney injury | Ischemia |
| Acute spinal cord injury | Ischemia |
| Amyothrophic lateral sclerosis | Autoinmmune |
| Asthma | Immune - inflammation |
| Autism | Unknown |
| Cognitive function/endarterectomy | Ischemia |
| Dry eye syndrome | Inflammation |
| Fragile X syndrome | Hereditary mental retardation |
| Gum disease | Inflammation |
| HIV – cognitive impairment | Viral - inflammation |
| Huntington's disease | Degenerative |
| Idiopathic pulmonary fibrosis | Unknown - inflammation |
| Ischemic stroke | Ischemia |
| Multisystem atrophy | Degenerative |
| Parkison's disease | Degenerative |
| Pneumothorax | Inflammation |
| Psychosis | Unknown |
| Rheumatoid arthritis | Inflammation |
| Schizophrenia | Unknown |
| Sclerosis cholangitis | Inflammation |
| Doxycycline | |
| Abdominal aneurysm | Inflammation - proteases |
| Alzheimer's disease | Degenerative |
| Bone loss | Hormonal imbalance |
| Brain hemorrhage | Ischemia |
| COPD | Inflammation |
| Coronary artery bypass | Ischemia |
| Diabetic retinopathy | Inflammation |
| Diabetic ulcers | Inflammation - healing |
| Lymphangioleiomyomatosis | Noncancerous tumor |
| Osteoarthritis | Inflammation |
| Postmyocardial infarction remodeling | Inflammation - healing |
| COL-3 | |
| Metastatic cancer | Cancer |
| Kaposi's sarcoma | Cancer |
| Recurring brain tumors | Cancer |
| Advanced solid tumors | Cancer |
Partial listing of clinical trials in different phases of execution as gathered from www.clinicaltrials.gov for minocycline (20/54 total), doxycycline (11/88), and the chemically modified tetracycline COL-3 (4/4). The listing only includes nonconventional uses.
Conclusions
Tetracyclines have been recognized slowly over time as a genre of drugs with interesting pleiotropic properties. Their accumulation in injured tissues makes them almost appear to act as a smart drug. The recognition by scientists and clinicians of these collection of properties and of the safety profile of this class of drugs has led to the implementation of clinical trials to explore their possible beneficial effects in the setting of a wide variety of diseases. Clinical trial efforts so far have been mostly isolated but, as a whole they would appear to require the formation of a consensus group that would lead to a more systematic understanding (and examination) of their therapeutic potential. As CMT have proven to generate useful derivatives with unique properties, a rational modification of this class of drugs may lead to the development of novel compounds with greater therapeutic potential and safety profiles. Indeed, such creative efforts are currently the focus of various research groups (84). However, it should be recognized that each member of the TTC family has both similar and more importantly, distinct properties from each other such as half life and lipophilicity. Scientists when anticipating their use, need to make extensive considerations towards the desired “preferred” action (e.g., antiapoptotic vs. antiprotease) and anticipated outcomes.
GRANTS
This work was supported by a NIH HL-43617 and AT-004277 to F. Villarreal, and G. Ceballos by a visiting professor CONACYT fellowship from Mexico.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ambrosio G, Becker LC, Hutchins GM, Weisman HF, Weisfeldt ML. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation 74: 1424–1433, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Baker PJ, Evans RT, Coburn RA, Genco RJ. Tetracycline and its derivatives strongly bind to and are released from the tooth surface in active form. J Periodontol 54: 580–585, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother 8: 713–720, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthon G, Brion M, Lambs L. Metal ion-tetracycline interactions in biological fluids. 2. Potentiometric study of magnesium complexes with tetracycline, oxytetracycline, doxycycline, and minocycline, and discussion of their possible influence on the bioavailability of these antibiotics in blood plasma. J Inorg Biochem: 1–18, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Blackwood RK, English AR. Structure-activity relationships in the tetracycline series. Adv Appl Microbiol; 13237–266, 1970 [Google Scholar]
- 6.Brion M, Lambs L, Berthon G. Metal ion-tetracycline interactions in biological fluids. Part 5 Formation of zinc complexes with tetracycline and some of its derivatives and assessment of their biological significance. Agents Actions 17: 229–242, 1975 [DOI] [PubMed] [Google Scholar]
- 7.Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali- burned rabbit corneas. Invest Ophthalmol Vis Sci 30: 1569–1575, 1989 [PubMed] [Google Scholar]
- 8.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med: 797–801, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol 30: 2281–2289, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101: 1833–1839, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Colaizzi JL, Klink PR. pH-Partition behavior of tetracyclines. J Pharm Sci 58: 1184–1189, 1969 [DOI] [PubMed] [Google Scholar]
- 12.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647–656, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res 89: 201–210, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Crout RJ, Lee HM, Schroeder K, Crout H, Ramamurthy NS, Wiener M, et al. The “cyclic” regimen of low-dose doxycycline for adult periodontitis: a preliminary study. J Periodontol 67: 506–514, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother 50: 3124–3131, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielsen CC, Wiggers H, Andersen HR. Increased amounts of collagenase and gelatinase in porcine myocardium following ischemia and reperfusion. J Mol Cell Cardiol 30: 1431–1442, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Desrochers PE, Mookhtiar K, Van Wart HE, Hasty KA, Weiss SJ. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem 267: 5005–5012, 1992 [PubMed] [Google Scholar]
- 18.Ding B, Price RL, Goldsmith EC, Borg TK, Yan X, Douglas PS, et al. Left ventricular hypertrophy in ascending aortic stenosis mice: anoikis and the progression to early failure. Circulation 101: 2854–2862, 2000 [DOI] [PubMed] [Google Scholar]
- 19.d'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem 250: 751–757, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the M.PTP model of Parkinson's disease. Proc Natl Acad Sci USA 98: 14669–14674, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duggar BM. Aureomycin: a product of the continuing search for new antibiotics. Ann NY Acad Sci 51: 177–181, 1948 [DOI] [PubMed] [Google Scholar]
- 22.Esterly NB, Koransky JS, Furey NL, Trevisan M. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol 120: 1308–1313, 1984 [PubMed] [Google Scholar]
- 23.Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, Baicu S, Mukherjee R, Manhaini M, Spinale FG. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol 281: H987–H994, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Fischer U, Jänicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 10: 76–100, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forloni G, Iussich S, Awan T, Colombo L, Angeretti N, Girola L, Bertani I, Poli G, Caramelli M, Grazia Bruzzone M, Farina L, Limido L, Rossi G, Giaccone G, Ironside JW, Bugiani O, Salmona M, Tagliavini Tetracyclines affect prion infectivity. Proc Natl Acad Sci USA 99: 10849–10854, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabler WL, Tsukuda N. The influence of divalent cations and doxycycline on iodoacetamide-inhibitable leukocyte adherence. Res Commun Chem Pathol Pharmacol 74: 131–140, 1991 [PubMed] [Google Scholar]
- 27.Garcia RA, Go KV, Villarreal FJ. Effects of timed administration of doxycycline or methylprednisolone on post-myocardial infarction inflammation and left ventricular remodeling in the rat heart. Mol Cell Biochem 300: 159–169, 2007 [DOI] [PubMed] [Google Scholar]
- 28.García RA, Pantazatos DP, Gessner CR, Go KV, Woods VL, Villarreal FJ. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol Pharmacol 67: 1128–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Goldman RA, Hasan T, Hall CC, Strycharz WA, Cooperman BS. Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry 22: 359–368, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Golub LM, Evans RT, McNamara TF, Lee HM, Ramamurthy NS. A non-antimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis-induced periodontitis in rats. Ann NY Acad Sci 732: 96–111, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, Ramamurthy NS. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodontal Res 18: 516–526, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Golub LM, McNamara TF, D'Angelo G, Greenwald RA, Ramamurthy NS. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J Dent Res 66: 1310–1314, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med 2: 297–321, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Golub LM, Sorsa T, Lee HM, Ciancio S, Sorbi D, Ramamurthy NS, Gruber B, Salo T, Konttinen YT. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol 22: 100–109, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89: 5547–5551, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwald RA, Golub LM, Ramamurthy NS, Chowdhury M, Moak SA, Sorsa T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone 22: 33–38, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Grenier D, Plamondon P, Sorsa T, Lee HM, McNamara T, Ramamurthy NS, Golub LM, Teronen O, Mayrand D. Inhibition of proteolytic, serpinolytic, and progelatinase-b activation activities of periodontopathogens by doxycycline and the non-antimicrobial chemically modified tetracycline derivatives. J Periodontol 73: 79–85, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Guignabert C, Taysse L, Calvet J, Planus E, Delamanche S, Galiacy S, d'Ortho MP. Effect of doxycycline on sulfur mustard-induced respiratory lesions in guinea pigs. Am J Physiol Lung Cell Mol Physiol 289: L67–L74, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Hash JH, Wishnick M, Miller PA. On the mode of action of the tetracycline antibiotics in Staphylococcus aureus. J Biol Chem 239: 2070–2078, 1964 [PubMed] [Google Scholar]
- 40.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res 61: 372–385, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, van Hinsbergh VW, Helaakoski T, Kainulainen T, Rönkä H, Tschesche H, Salo T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem 272: 31504–31509, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Hinrichs W, Kisker C, Düvel M, Müller A, Tovar K, Hillen W, et al. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science 264: 418–420, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Hoeben D, Burvenich C, Heyneman R. Antibiotics commonly used to treat mastitis and respiratory burst of bovine polymorphonuclear leukocytes. J Dairy Sci 81: 403–410, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Holman BL. Radionuclide methods in the evaluation of myocardial ischemia and infarction. Circulation 53, Suppl: I112–I119, 1976 [PubMed] [Google Scholar]
- 45.Holman BL, Zweiman FG. Time course of 99mTc(Sn)-tetracycline uptake in experimental acute myocardial infarction. J Nucl Med 16: 1144–1146, 1975 [PubMed] [Google Scholar]
- 46.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem 73: 87–106, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res 54: 277–285, 1984 [DOI] [PubMed] [Google Scholar]
- 48.Jonat C, Chung FZ, Baragi VM. Transcriptional downregulation of stromelysin by tetracycline. J Cell Biochem 60: 341–347, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Jones D. Reactive oxygen species and rosacea. Cutis 74, Suppl 3: 17–20, 32–34, 2004 [PubMed] [Google Scholar]
- 50.Joshi NJ, Miller D. Doxycycline revisited. Arch Intern Med 157: 1421–1428, 1997 [PubMed] [Google Scholar]
- 51.Korting HC, Schöllman C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol 22: 187–194, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem 94: 819–827, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Lambs L, Brion M, Berthon G. Metal ion-tetracycline interactions in biological fluids. Part 3: Formation of mixed-metal ternary complexes of tetracycline, oxytetracycline, doxycycline and minocycline with calcium and magnesium, and their involvement in the bioavailability of these antibiotics in blood plasma. Agents Actions 14: 743–750, 1984 [DOI] [PubMed] [Google Scholar]
- 54.León H, Baczkó I, Sawicki G, Light PE, Schulz R. Inhibition of matrix metalloproteinases prevents peroxynitrite-induced contractile dysfunction in the isolated cardiac myocyte. Br J Pharmacol 153: 676–683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy SB, McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun 56: 1060–1068, 1974. [DOI] [PubMed] [Google Scholar]
- 56.McNamara TF, Golub LM, D'Angelo G, Ramamurthy NS. The synthesis and characterization of non-antimicrobial chemically-modified tetracycline (CMT) (Abstract). J Dent Res 65: IADR no. 515, 1986 [Google Scholar]
- 57.Michaelis J, Vissers MC, Winterbourn CC. Human neutrophil collagenase cleaves alpha 1-antitrypsin. Biochem J 270: 809–814, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ 10: 108–116, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Näslund U, Häggmark S, Johansson G, Marklund SL, Reiz S, Oberg A. Superoxide dismutase and catalase reduce infarct size in a porcine myocardial occlusion-reperfusion model. J Mol Cell Cardiol 18: 1077–1084, 1986 [DOI] [PubMed] [Google Scholar]
- 61.Nelson ML. Chemical and biological dynamics of tetracyclines. Adv Dent Res 12: 5–11, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg 68: 1905–1912, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Pelle MT, Crawford GH, James WD. Rosacea II Therapy. J Am Acad Dermatol 51: 499–512, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev 9: 63–79, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Pruzanski W, Greenwald RA, Street IO, La-leberte F, Stefanski E, vadas P. Inhibition of enzymatic activity of phospholipase A2 by minocycline and doxycycline. Biochem Pharmacol 44: 1165–1170, 1992 [DOI] [PubMed] [Google Scholar]
- 66.Ramamurthy NS, Vernillo AT, Greenwald RA, Lee HM, Sorsa T, Golub LM, Rifkin BR. Reactive oxygen species activate and tetracyclines inhibit rat osteoblast collagenase. J Bone Miner Res 8: 1247–1253, 1993 [DOI] [PubMed] [Google Scholar]
- 67.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation 99: 3063–3070, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci 68: 799–814, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Romero-Perez D, Fricovsky E, Yamasaki KG, Griffin M, Barraza-Hidalgo M, Dillmann W, Villarreal F. Cardiac uptake of minocycline and mechanisms for in vivo cardioprotection. J Am Coll Cardiol 52: 1086–1094, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan ME, Usman A, Ramamurthy NS, Golub LM, Greenwald RA. Excessive matrix metalloproteinase activity in diabetes: inhibition by tetracycline analogues with zinc reactivity. Curr Med Chem 8: 305–316, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Sabri A, Alcott SG, Elouardighi H, Pak E, Derian C, Andrade-Gordon P, Kinnally K, Steinberg SF. Neutrophil cathepsin G promotes detachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J Biol Chem 278: 23944–23954, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene 23: 2861–2874, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 8: 1393–1401, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation 112: 544–552, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Scarabelli TM, Stephanou A, Pasini E, Gitti G, Townsend P, Lawrence K, Chen-Scarabelli C, Saravolatz L, Latchman D, Knight R, Gardin J. Minocycline inhibits caspase activation and reactivation, increases the ratio of XIAP to smac/DIABLO, and reduces the mitochondrial leakage of cytochrome C and smac/DIABLO. J Am Coll Cardiol 43: 865–874, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Semenkov YuP, Makarov EM, Makhno VI, Kirillov SV. Kinetic aspects of tetracycline action on the acceptor (A) site of Escherichia coli ribosomes. FEBS Lett 144: 125–129, 1982 [DOI] [PubMed] [Google Scholar]
- 77.Smith GN, Brandt KD, Mickler EA, Hasty KA. Inhibition of recombinant human neutrophil collagenase by doxycycline is pH dependent. J Rheumatol 24: 1769–1773, 1997 [PubMed] [Google Scholar]
- 78.Smith GN, Mickler EA, Hasty KA, Brandt KD. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthritis Rheum 42: 1140–1146, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Sorsa T, Ding Y, Salo T, Lauhio A, Teronen O, Ingman T, Ohtani H, Andoh N, Takeha S, Konttinen YT. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Ann NY Acad Sci 732: 112–131, 1994 [DOI] [PubMed] [Google Scholar]
- 80.Sorsa T, Konttinen YT, Lindy O, Suomalainen K, Ingman T, Saari H, Halinen S, Lee HM, Golub LM, Hall J. Doxycycline protects serum alpha-1-antitrypsin from human neutrophil collagenase. Agents Actions Suppl 39: 225–229, 1993 [DOI] [PubMed] [Google Scholar]
- 81.Sorsa T, Lindy O, Konttinen YT, Suomalainen K, Ingman T, Saari H, Halinen S, Lee HM, Golub LM, Hall J. Doxycycline in the protection of serum alpha-1-antitrypsin from human neutrophil collagenase, and gelatinase. Antimicrob Agents Chemother Mar 37: 592–594, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 90: 520–530, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Steinberg J, Halter J, Schiller H, Gatto L, Carney D, Lee H, Golub L, Nieman G. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock 24: 348–356, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Sun C, Wang Q, Brubaker JD, Wright PM, Lerner CD, Noson K, et al. A robust platform for the synthesis of new tetracycline antibiotics. J Am Chem Soc 130: 17913–17927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Takahashi M, Altschmied L, Hillen W. Kinetic and equilibrium characterization of the Tet repressor-tetracycline complex by fluorescence measurements. Evidence for divalent metal ion requirement and energy transfer. J Mol Biol 187: 341–348, 1986 [DOI] [PubMed] [Google Scholar]
- 87.Thong YH, Ferrante A. Inhibition of mitogen-induced human lymphocyte proliferative responses by tetracycline analogues. Clin Exp Immunol 35: 443–446, 1979 [PMC free article] [PubMed] [Google Scholar]
- 88.Thorburn A. Death receptor-induced cell killing. Cell Signal 16: 139–144, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Tritton TR. Ribosome-tetracycline interactions. Biochemistry 16: 4133–4138, 1977 [DOI] [PubMed] [Google Scholar]
- 90.Villarreal F, Omens J, Dillmann W, Risteli J, Nguyen J, Covell J. Early degradation and serum appearance of type I collagen fragments after myocardial infarction. J Mol Cell Cardiol 36: 597–601, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Walters JD. Characterization of minocycline transport by human neutrophils. J Periodontol 7: 1964–1968, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Wei Q, Wang C, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Wang P, Chen H, Qin H, Sankarapandi S, Becher MW, Wong PC, Zweier JL. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc Natl Acad Sci USA 95: 4556–4560, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 106: 1543–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci USA 100: 10483–10487, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Webster GF, Toso SM, Hegemann L. Inhibition of a model of in vitro granuloma formation by tetracyclines and ciprofloxacin: involvement of protein kinase C. Arch Dermatol 130: 748–752, 1994 [PubMed] [Google Scholar]
- 97.Yamaguchi A, Udagawa T, Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem 265: 4809–4813, 1990 [PubMed] [Google Scholar]
- 98.Yang Q, Nakkula RJ, Walters JD. Accumulation of ciprofloxacin and minocycline by cultured human gingival fibroblasts. J Dent Res 81: 836–840, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshida T, Watanabe M, Engelman DT, Engelman RM, Schley JA, Maulik N, Ho YS, Oberley TD, Das DK. Transgenic mice overexpressing glutathione peroxidase are resistant to myocardial ischemia reperfusion injury. J Mol Cell Cardiol 28: 1759–1767, 1996 [DOI] [PubMed] [Google Scholar]
- 100.Yu LP, Smith GN, Hasty KA, Brandt KD. Doxycycline inhibits type XI collagenolytic activity of extracts from human osteoarthritic cartilage and of gelatinase. J Rheumatol 18: 1450–1452, 1991 [PubMed] [Google Scholar]
- 101.Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95: 15769–15774, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 293: 2003–2011, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Zhu S, Stavrovskaya IG, Drozda M, Kim BYS, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417: 74–78, 2002 [DOI] [PubMed] [Google Scholar]