Abstract
Recent developments in tissue engineering approaches frequently revolve around the use of three-dimensional scaffolds to function as the template for cellular activities to repair, rebuild and regenerate damaged or lost tissues. While there are several biomaterials to select as three-dimensional scaffolds, it is generally agreed that a biomaterial to be used in tissue engineering needs to possess certain material characteristics such as biocompatibility, suitable surface chemistry, interconnected porosity, desired mechanical properties and biodegradability. The use of naturally derived polymers as three-dimensional scaffolds has been gaining widespread attention owing to their favourable attributes of biocompatibility, low cost and ease of processing. This paper discusses the synthesis of various polysaccharide-based, naturally derived polymers, and the potential of using these biomaterials to serve as tissue engineering three-dimensional scaffolds is also evaluated.
In this study, naturally derived polymers, specifically cellulose, chitosan, alginate and agarose, and their composites, are examined. Single-component scaffolds of plain cellulose, plain chitosan and plain alginate as well as composite scaffolds of cellulose–alginate, cellulose–agarose, cellulose–chitosan, chitosan–alginate and chitosan–agarose are synthesized, and their suitability as tissue engineering scaffolds is assessed. It is shown that naturally derived polymers in the form of hydrogels can be synthesized, and the lyophilization technique is used to synthesize various composites comprising these natural polymers. The composite scaffolds appear to be sponge-like after lyophilization. Scanning electron microscopy is used to demonstrate the formation of an interconnected porous network within the polymeric scaffold following lyophilization. It is also established that HeLa cells attach and proliferate well on scaffolds of cellulose, chitosan or alginate. The synthesis protocols reported in this study can therefore be used to manufacture naturally derived polymer-based scaffolds as potential biomaterials for various tissue engineering applications.
Keywords: natural polymers, composite scaffolds, cellulose, chitosan, alginate, agarose
1. Introduction
Traditionally, tissue engineering techniques rely on the harvesting of tissues or organs from various sources. Depending on the sources, the harvesting can be categorized as either autologous (from one’s own tissues), allogenic (from other human donors) or xenogenic (from another species) (Wei & Ma 2004; Ahsan et al. 2008). The use of these conventional grafts, however, has also encountered serious problems and limitations. For example, the use of autografts and allografts are associated with donor shortage and donor site morbidity, whereas allografts and xenografts have the risk of transmitting harmful diseases and also triggering undesirable immune responses (Bonfiglio & Jeter 1972; Burg et al. 2000). Hence, there has been considerable research ongoing in recent years to develop replacement materials that can substitute for these grafts to eliminate the aforementioned problems.
The current trend in tissue regeneration revolves around the implantation of three-dimensional scaffolds. The scaffolds serve as a three-dimensional specimen to provide for initial cell attachment and subsequent tissue formation (Hutmacher et al. 2001). The ability either to repair damaged tissues or to regenerate defective organs using an implanted three-dimensional scaffold would eliminate not only the donor shortage problems but also the inevitable need for immunosuppression treatments that tend to be necessary. Ideally, a three-dimensional scaffold should have the following characteristics (Hutmacher et al. 2001; Varghese & Elisseeff 2006): (i) possess excellent biocompatibility and bioactivity to promote cell growth; (ii) exhibit three-dimensional architecture and display a highly porous microstructure with an interconnected pore network to induce good flow transport characteristics of nutrients and metabolic waste; (iii) possess biodegradable or bioresorbable characteristics with a controlled optimum degradation or resorption rate to match tissue growth without releasing cytotoxic degradation products; (iv) display suitable surface chemistry for cell attachment, proliferation and differentiation; (v) possess the desired mechanical properties to match those of the tissues at the site of implantation; and (vi) be easily processed to form a variety of shapes and sizes. Hence, the selection of a three-dimensional scaffold is very significant in determining how the scaffold will behave in various tissue engineering applications.
(a). Naturally derived polymers
Naturally derived polymers, as their name implies, are derived from natural sources. Naturally derived polymers are biocompatible and are typically composed of a polymeric network that can contain up to 99 per cent or higher water content. As a result, they have typically been referred to as ‘hydrogels’, and their swelling capability in water allows them to exhibit an environment that resembles the highly hydrated state of natural tissues (Ratner & Bryant 2004). Various gelation mechanisms exist depending on the polymer selected: gelation in response to temperature or pH change, ionic cross-linking, solvent exchange or crystallization, and simply modifying the viscosity of the polymer solution (Gutowska et al. 2001). Even though naturally derived polymers are characterized by having batch-to-batch variations and poor mechanical properties (Yang et al. 2001), they are also readily available, inexpensive and easy to fabricate into hydrogels, making them appealing choices for scaffolds. Among all the naturally derived polymers, polysaccharides, composed of sugar-ring building blocks, are emerging as a front runner in various tissue engineering applications. Commonly used polysaccharides include cellulose, chitosan, alginate and agarose.
Cellulose and its derivatives are the most abundant naturally occurring polysaccharide found in nature, having glucose-based repeat units connected by β-1,4-glucosidic linkages (Entcheva et al. 2004). Cellulose and its derivatives are biocompatible (Esposito et al. 2005) and generally known to possess desirable properties such as high water-holding capacity, high crystallinity, fine fibre network, ease of mouldability and high tensile strength (Svensson et al. 2005). Cellulose is biodegradable in nature by microbial or fungal enzymes that can break down the glucosidic linkages, but in animals or humans, biodegradation is limited or absent owing to the lack of these enzymes (Helenius et al. 2006). Commonly used cellulose derivatives include carboxymethylcellulose (CMC), hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), hydroxypropyl methylcellulose (HPMC), cellulose acetate and bacterial cellulose (Guo et al. 1998; Entcheva et al. 2004; Helenius et al. 2006). Cellulose derivatives have been used frequently in applications such as gel base, film coatings, binders, bioadhesives, controlled release of drugs, thickeners and stabilizers, tissue regeneration and treatment of oedema (Sebert et al. 1994; Guo et al. 1998; Sannino et al. 2003; Entcheva et al. 2004; Svensson et al. 2005).
Chitosan is derived from chitin, which is found in the exoskeleton of marine crustaceans such as shrimps and crabs, as well as insects and the cell walls of fungi (Hejazi & Amiji 2003; Issa et al. 2005). It is derived from chitin through a deacetylation process to obtain a linear structure of glucosamine and N-acetyl-glucosamine linked in a β-1,4 manner (Di Martino et al. 2005). Chitosan is soluble at pH lower than 5.5, thus solvents such as acetic acid and hydrochloric acid are often used so as to dissolve chitosan. Chitosan forms gels either by raising the pH to 6 or higher (Suh & Matthew 2000) or by interacting with a variety of divalent and polyvalent anions (Hejazi & Amiji 2003). Chitosan is known to be biocompatible, biodegradable, non-toxic and biofunctional (Zhang & Zhang 2001). The applications of chitosan include drug delivery (Li & Xu 2002; Zhang & Zhang 2002; Hejazi & Amiji 2003), growth factor encapsulation (Kim et al. 2003) and gene delivery by forming complexes between the cationic chitosan and negatively charged DNA (Lee et al. 1998; Roy et al. 1999).
Alginate is a polysaccharide derived from brown algae, certain seaweeds or bacteria. It is a linear polysaccharide copolymer of (1,4)-linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) monomers (Drury & Mooney 2003), and its structure consists of blocks of M or G monomers. The composition and arrangement of the M and G monomers determine the structural properties of alginate, as alginate richer in the blocks of G monomers have a higher elastic modulus and higher solute diffusivity (Ehrenfreund-Kleinman et al. 2006). Alginate gels readily in the presence of divalent ions such as Ca2+, Ba2+ and Sr2+ (Lin & Yeh 2004) via ionic cross-linking. Alginate has been used in the food industry as thickening and emulsifying agents in products such as ice creams, beer and cake mixtures (Ma 2006). Alginate has also been used in various biomedical applications, including drug, antibody or growth factor delivery (Perets et al. 2003; Sivakumar & Rao 2003; Ma 2006), cell encapsulation and seeding (Glicklis et al. 2000; Gerecht-Nir et al. 2004), and gene delivery in plants and mammals in the form of microspheres (Sone et al. 2002; Higashi et al. 2004). Alginate scaffolds have also been used in conjunction with another weaker material to reinforce the mechanical properties (Miralles et al. 2001; Li et al. 2005).
Agarose is a polysaccharide with alternating copolymers of (1,4)-linked 3,6-anhydro-α-l-galactose and (1,3)-linked β-d-galactose (Balgude et al. 2001). Its gelling conditions are temperature dependent since it is soluble in water above 65°C and gels upon cooling to temperatures between 37 and 40°C (Balgude et al. 2001; Hutmacher et al. 2001). Its gelation mechanism relies on the sol–gel transition (sol phase: flowing fluid; gel phase: non-flowing fluid, measured on an experimental time scale) upon lowering of temperature (Jeong et al. 2002). Agarose is commonly used in molecular biology as the porous medium for electrophoresis. Most studies conducted to date using agarose as a scaffold involve cartilage repair (Hutmacher et al. 2001; Aufderheide & Athanasiou 2005; Hu & Athanasiou 2005). The thermosensitive characteristic of agarose has been used to engineer an injectable hydrogel for the formation of cartilage in vivo (Xu et al. 2005).
(b). Composite scaffolds
In addition to single-component scaffolds, composites of various naturally derived polymers are also investigated in this paper. Each individual natural polymer has its own characteristic advantages and disadvantages. It is commonly accepted that composite materials, or hybrids, often show an excellent balance between the strengths and weaknesses, and overall exhibit improved characteristics compared with the individual components (Liu & Ma 2004). In order to eliminate undesired characteristics and exploit the advantages of the individual polymers, various composite scaffolds comprising two or more polymers have often been considered. For example, the stability of polymers that are mechanically weaker can be reinforced by fabricating composites containing mechanically stronger polymers. Similarly, polymers that possess poor cell proliferation or migration properties can be remedied with the incorporation of a more bioactive polymeric phase.
(c). Major goals
This paper aims to demonstrate novel synthesis techniques to fabricate both single-component scaffolds as well as composite scaffolds comprising various naturally derived polymers, including cellulose, chitosan, alginate and agarose. The synthesis protocols of the scaffolds will be discussed in detail, exploiting the unique gelation mechanisms of each natural polymer, and the scaffolds will be evaluated for their suitability as tissue engineering scaffolds.
2. Materials and methods
(a). Materials
Sodium CMC (low viscosity, viscosity 50–200 cP at 4 wt% in H2O), HEC (75–150 cP at 5 wt% in H2O), divinyl sulfone (DVS, 97% purity) and potassium hydroxide (KOH, greater than 85% ACS reagent grade) were purchased from Sigma Aldrich (St Louis, MO, USA). Acetone (certified ACS grade) and acetic acid (glacial, certified ACS grade) were purchased from Fisher Scientific (Pittsburgh, PA, USA). Two Ultrasans with a degree of deacetylation (DDA) of 81 per cent and 91 per cent were purchased from Biosyntech Inc. (Quebec, Canada). Chitosan from crab shell (practical grade, approx. 75% DDA, 200.00 cP), low-molecular-weight chitosan (approx. 83% DDA, 20.00 cP), β-glycerolphosphate disodium salt pentahydrate (β-GP, greater than 98%) and calcium chloride (CaCl2⋅2H2O, 99%) were also purchased from Sigma Aldrich. Sodium hydroxide (NaOH) was purchased from Mallinckrodt Baker Inc. (Phillipsburg, NJ, USA). Alginate, sodium salt, was also purchased from Fisher Scientific. Agarose, UltraPure, was obtained from Invitrogen (Carlsbad, CA, USA). All the reagents were used as received without further modification or purification.
(b). Synthesis of cellulose hydrogels
Cellulose-based scaffolds were synthesized by following previously described synthesis protocols (Anbergen & Oppermann 1990; Esposito et al. 1996; Capitani et al. 2000), using a mixture of CMC and HEC cross-linked with DVS at an alkaline pH. Briefly, CMC and HEC powders with a 3:1 weight ratio were dissolved in doubly distilled water (ddH2O, 18.2 MΩ cm resistivity) to form a 6–8 wt% solution. Then, a 1 M KOH solution was added to the CMC/HEC mixture such that the overall KOH concentration in the CMC/HEC mixture is 0.02 M. The pH of the solution after the addition of KOH was approximately 12.5. The CMC/HEC/KOH mixture was then cooled to 0°C in an ice bath while the mixture was kept under stirring. Then, DVS, the cross-linking agent, was added drop by drop into the CMC/HEC/KOH mixture solution such that the final DVS concentration in the mixture was between 10 and 40 mol m−3. The final solution mixture was left undisturbed at room temperature for 24 h for the cross-linking and gelation reaction to occur. Since DVS is known to be toxic to cells, and the pH of KOH was exceedingly basic, the cellulose gel was immersed in ddH2O to rinse away any excess, unreacted DVS and KOH. The ddH2O was exchanged several times to ensure the complete removal of DVS and KOH. An image of the prototype cellulose hydrogel is shown in figure 1.
Figure 1.
Cellulose-based hydrogel synthesized with a mixture of CMC and HEC at a weight ratio of 3:1.
In order to create the microporosity essential for a tissue engineering scaffold within the polymeric structure, the cellulose hydrogels were immersed into acetone. Acetone, which is a solvent for ddH2O but not the cellulose polymer, allowed the extraction of water from within the cellulose polymer, thereby inducing the microporosity. The acetone immersion step for water extraction was repeated at least three times. The scaffolds were then kept under vacuum to remove traces of residual acetone. The samples were then stored in a desiccator prior to evaluation.
(c). Synthesis of chitosan hydrogels
Chitosan-based scaffolds were synthesized taking into consideration the precipitated form of the chitosan phase upon increasing the pH above 5.5. Briefly, chitosan powders were dissolved in 0.1 N glacial acetic acid to form a 2 per cent solution. The chitosan solution was sterilized by autoclaving. To gel the chitosan, 1 N NaOH was added drop by drop to completely cover the chitosan solution and to induce the gelation of chitosan by raising the pH. The chitosan gels were then rinsed with ddH2O at least three times to rinse away excess NaOH and stored at 4°C prior to evaluation.
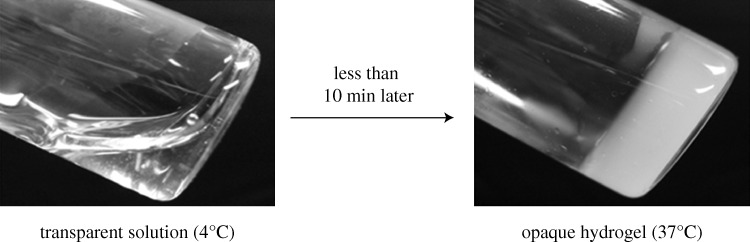
A commercially available chitosan, Ultrasan, which possesses thermosensitive gelation properties with the introduction of β-GP to increase the pH to nearly neutral (approx. 7.2), was used as a comparison in this study. At low temperatures (approx. 4°C), the Ultrasan solution remains liquid, whereas at the physiological temperature of 37°C, an irreversible gelation process occurs, transforming the chitosan solution into an opaque gel. Ultrasan scaffolds were synthesized according to previously published synthesis protocols (Ruel-Gariepy et al. 2000; Molinaro et al. 2002). Briefly, Ultrasan powders (81% or 91% DDA) were dissolved in ddH2O to form a 1.5 per cent or 2 per cent solution, and the Ultrasan solution was sterilized by autoclaving. A 45 per cent β-GP solution was prepared and sterilized using a 0.22 μm sterile filter. Both the β-GP solution and the Ultrasan solution were chilled to 4°C, and the β-GP solution was added drop by drop into the Ultrasan solution while under stirring. The Ultrasan/β-GP mixture was stored in a 37°C incubator to allow the gelation of the Ultrasan to occur. The thermosensitive transition of Ultrasan from the liquid to gel phase can be seen from figure 2.
Figure 2.
Images showing the thermosensitive transition of Ultrasan from liquid to gel as a function of time.
(d). Synthesis of alginate hydrogels
Alginate-based scaffolds were synthesized taking into account the gelation mechanism of alginate in the presence of divalent ions such as Ca2+. Alginate powders were dissolved in ddH2O to form 1.5–2.0% alginate solutions. A 0.03 M CaCl2 cross-linking solution was added drop by drop into the alginate solution until the CaCl2 solution completely covers the alginate. The alginate scaffold was immersed in CaCl2 overnight at 4°C, followed by immersion in ddH2O to rinse away excess CaCl2. An image of the alginate hydrogel cross-linked by divalent Ca2+ ions is shown in figure 3.
Figure 3.
Alginate gel formed by the cross-linking of alginate solution with CaCl2.
(e). Syntheses of natural polymer-based composite scaffolds via lyophilization
In addition to the individual polymers, various composites of naturally derived polymers were synthesized, including cellulose–alginate, cellulose–agarose, cellulose–chitosan, chitosan–alginate and chitosan–agarose. Note that the chitosan used in these composites was the chitosan purchased from Sigma Aldrich, not Ultrasan. In order to synthesize composite scaffolds with two distinct natural polymer components, the process of lyophilization was used after freezing the composites at −80°C for 24 h and lyophilizing them for 72 h using a Labconco FreeZone 4.5 freeze-dry system (Labconco Corp., Kansas City, MO, USA). The sublimation of ice crystals into the vapour phase was expected to result in macroporous structures suitable for cell growth and migration and nutrient transport. The synthesis protocols for these composite scaffolds are detailed in the following subsections.
(i). Synthesis of cellulose–alginate composite scaffolds
Cellulose–alginate composite scaffolds were synthesized in the following manner. First, CMC and HEC powders with a weight ratio of 3:1 were dissolved in ddH2O to form a 2 wt% solution. Alginate powders were dissolved in another quantity of ddH2O to form a 2 per cent solution (the weight ratio of cellulose to alginate being 1:1). After the complete dissolution of both polymers, an equal volume of the alginate solution was added to the cellulose mixture. Then, a 1 M KOH solution was added to the CMC/HEC/alginate mixture such that the overall KOH concentration in the mixture was 0.02 M. The mixture was cooled to 0°C, and while under stirring, DVS was added drop by drop to the mixture such that the overall DVS concentration was between 10 and 40 mol m−3. After stirring the solution for approximately 30 min, the final mixture was left undisturbed at room temperature for 24 h for the gelation reaction to occur. The composite gel was frozen at −80°C, lyophilized for 72 h, and the dried scaffold was immersed in a 0.03 M CaCl2 solution for the cross-linking of alginate to occur. The resulting cellulose–alginate composite was again frozen at −80°C and lyophilized for 72 h until all solvents were removed. The final dried cellulose–alginate composite was stored in a desiccator prior to evaluation.
(ii). Synthesis of cellulose–agarose composite scaffolds
Cellulose–agarose composite scaffolds were synthesized in the following manner. First, CMC and HEC powders with a weight ratio of 3:1 were dissolved in ddH2O to form a 2 wt% solution. Agarose powders were dispersed into another quantity of ddH2O and the suspension was heated in a conventional microwave oven for 1 min to allow the dissolution of agarose, with a final agarose concentration of 1.5 per cent (the weight ratio of cellulose to agarose being 4 : 3). After the complete dissolution of both polymers, an equal volume of the agarose solution was added to the CMC/HEC mixture. The combined solution was quickly stirred before it cooled down to ensure homogeneity of the mixture and left undisturbed to allow the gelation of the agarose phase to occur upon cooling. The cellulose–agarose composite gel was frozen at −80°C for 24 h, lyophilized for 72 h and the dried scaffold was immersed into a KOH/DVS mixture containing an overall KOH concentration of 0.02 M and an overall DVS concentration of 10–40 mol m−3. The composite was again left undisturbed for 24 h to allow the cross-linking of CMC/HEC by DVS. The cellulose–agarose composite was again frozen at −80°C for 24 h and lyophilized for 72 h until all solvents were removed. The final dried cellulose–agarose composite was stored in a desiccator prior to evaluation.
(iii). Synthesis of cellulose–chitosan composite scaffolds
Cellulose–chitosan composite scaffolds were synthesized in the following manner. First, CMC and HEC powders with a weight ratio of 3:1 were dissolved in ddH2O to form a 2 wt% solution. Chitosan powders were dissolved in a 0.1 N glacial acetic acid solution to allow the dissolution of chitosan at a concentration of 1.5 per cent (the weight ratio of cellulose to chitosan being 4:3). After the complete dissolution of both polymers, an equal volume of the chitosan solution was added to the CMC/HEC mixture. The CMC/HEC/chitosan mixture was cooled to 0°C, and while under stirring, DVS was added drop by drop to the mixture such that the overall DVS concentration was between 10 and 40 mol m−3. Then, a 1 M KOH solution was added drop by drop to the CMC/HEC/chitosan solution such that the overall KOH concentration in the mixture was 0.02 M. The drop-wise addition of KOH into the mixture resulted in an increase in pH and subsequently the gelation of the chitosan phase. A blender was used to thoroughly mix the mixture to ensure homogeneity. After blending, the mixture was left undisturbed at room temperature for 24 h for the gelation of cellulose to occur. The cellulose–chitosan composite was then frozen at −80°C for 24 h and lyophilized for 72 h until all solvents were removed. The final dried cellulose–chitosan composite was stored in a desiccator prior to evaluation.
(iv). Synthesis of chitosan–alginate composite scaffolds
Chitosan–alginate composite scaffolds were synthesized in the following manner. Chitosan powders were dissolved in 0.1 N glacial acetic acid to form a 1.5 per cent solution. Alginate powders were dissolved in a solution of 1 M NaOH to form a 2 per cent solution (the weight ratio of chitosan to alginate being 3:4). The chitosan and the alginate solutions were mixed together, and the increase in pH resulted in the gelation of the chitosan phase. Glacial acetic acid was added drop by drop into the polymer mixture to adjust the pH of the mixture to approximately 7.4. The mixture was then homogenized in a blender for 1 h to form a mixture with a 2 per cent final polymer content. The mixture was frozen at −80°C for 24 h, and then lyophilized for 72 h. The dried scaffold was then immersed into a 0.03 M CaCl2 solution for the cross-linking of alginate to occur. The composite was rinsed with ddH2O three times to remove unreacted CaCl2, frozen at −80°C for 24 h and lyophilized for 72 h. The final chitosan–alginate composite was stored in a desiccator prior to evaluation.
(v). Synthesis of chitosan–agarose composite scaffolds
Chitosan–agarose composite scaffolds were synthesized in the following manner. Chitosan powders were dissolved in 0.1 N glacial acetic acid to form a 2 per cent solution. Agarose powders were dispersed in ddH2O and the suspension was heated in a conventional microwave oven for 1 min to allow the dissolution of agarose, with a final agarose concentration of 1.5 per cent (the weight ratio of chitosan to agarose being 4:3). The chitosan and the agarose solutions were mixed together and left undisturbed for the gelation of agarose to occur while the mixture cooled down. Then, NaOH was added drop by drop into the polymer mixture to adjust the pH of the mixture to approximately 7.4 and to induce the gelation of the chitosan phase. The mixture was then homogenized in a blender for 1 h, frozen at −80°C for 24 h and lyophilized for 72 h. The final chitosan–agarose composite was stored in a desiccator prior to evaluation.
(f). Scanning electron microscopy
Natural polymer-based composite scaffolds were synthesized as described above in their respective sections and scanning electron microscopy (SEM) was used to observe the morphology and the microstructure of the scaffolds. All of the samples for SEM were dried using the lyophilization technique and stored in a desiccator prior to examination under the SEM. The samples were mounted onto double-sided carbon tapes and sputter coated with palladium (Pd) using a sputter coater (Cressington 108A, Watford, UK). SEM images were collected using a Philips XL30 field emission gun SEM (FEI Company, Hillsboro, OR, USA).
(g). Cell culture and cell seeding on scaffolds
HeLa cells were used throughout the study owing to the simplicity of handling and culturing these cells. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Biowhittaker, Walkersville, MD, USA) supplemented with 10 per cent foetal bovine serum (Atlantic Biological, Lawrenceville, GA, USA) and 2 per cent penicillin–streptomycin (Gibco, Grand Island, NY, USA), and maintained in a 37°C incubator (Forma Scientific, Marietta, OH, USA) with 5 per cent CO2. HeLa cells were seeded at a cell density of 160 000 cells per well in 12-well plates.
In preparation for cell-based experiments, the scaffolds, including cellulose, chitosan and alginate hydrogels, underwent an incubation step prior to cell seeding. The scaffolds were rinsed with phosphate-buffered saline (PBS; Biowhittaker, Walkersville, MD, USA) and then immersed in DMEM, the same culture medium as used for the HeLa cells. The DMEM was discarded and replenished at least three times prior to cell seeding. HeLa cells cultured on the scaffolds were observed under a Nikon TS-100 optical microscope (Nikon Inc., Melville, NY, USA).
3. Results and discussion
(a). Characterization of natural polymer-based scaffolds and composite scaffolds
It is well known that hydrogels often appear transparent or semi-translucent, as shown in figures 1–3, and can hold up to more than 90 per cent water by weight compared with the polymeric component. The fact that hydrogels are composed of such high water content will probably be a significant factor since they resemble the highly hydrated state of natural tissues found in all living organisms. In addition to the hydrogels, in order to synthesize the composite scaffolds in this study, the lyophilization technique was employed not only to fabricate a scaffold containing two distinctly different polymers in both materials properties and gelation mechanisms, but also to create the microporosity within the scaffold by extracting the water phase to leave behind the porosity. Digital images of a plain cellulose scaffold and of the composite scaffolds based on natural polymers of cellulose, chitosan, alginate and agarose are shown in figure 4. As a result of the lyophilization process, the scaffolds appeared to be sponge-like, with porosity seen both on the surface and within the three-dimensional structure of the scaffolds.
Figure 4.
Images of scaffolds of (a) pure cellulose, and composite scaffolds of (b) cellulose–alginate, (c) cellulose–agarose, (d) chitosan–cellulose, (e) chitosan–agarose and (f) chitosan–alginate.
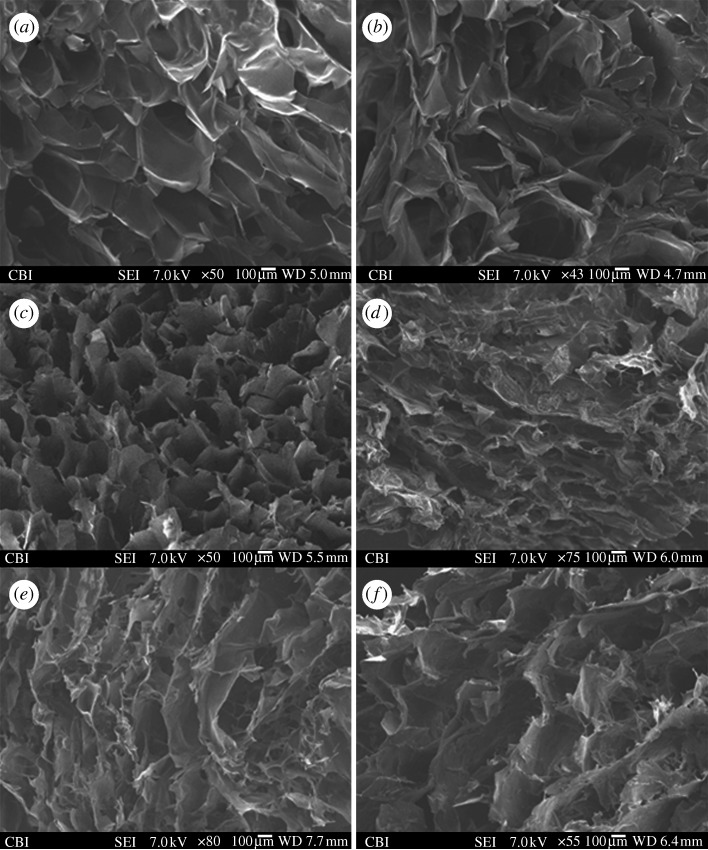
The SEM images of the cross sections of a plain cellulose scaffold and the composite scaffolds based on natural polymers of cellulose, chitosan, alginate and agarose are shown in figure 5. In general, the morphology and structure of the scaffolds appeared to be very similar, with all the scaffolds showing a sponge-like structure. However, depending on the materials used, the polymer concentration and the gelation mechanism of each individual polymer, the composite scaffolds also varied in appearance. Nevertheless, the porous structure within the scaffolds could be clearly observed. Depending on the composite scaffolds, the pore size can range from as small as 50–100 μm (figure 5d,e) in the case of chitosan–cellulose and chitosan–alginate to as large as 300–500 μm (figure 5a–c) for cellulose and cellulose–agarose. Pore sizes within these ranges are suitable for the infiltration of cells into the biomaterials and nutrient/waste transport when used as a tissue engineering scaffold. The pore size distribution within the same composite scaffold could also vary, which was a direct result of how the scaffolds were synthesized and how a particular region of the scaffolds was subjected to lyophilization, namely the freezing and sublimation direction of the frozen solvent. Overall, the morphology and structure of the composite scaffolds reflected the very basic requirement that a biomaterial scaffold must possess for use in tissue engineering applications.
Figure 5.
Cross-sectional SEM images of (a,b) cellulose scaffolds, (c) cellulose–agarose, (d) chitosan–cellulose, (e) chitosan–alginate and (f) chitosan–agarose, showing the porosity induced by lyophilization.
(b). Cell culture on cellulose hydrogels
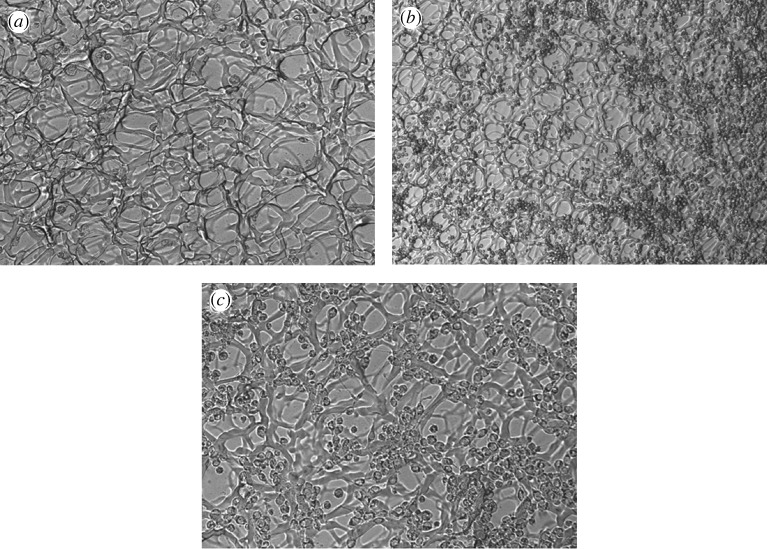
Optical microscopy images were taken showing HeLa cells cultured on cellulose hydrogels, as shown in figure 6. The cross-linked structure of CMC and HEC by DVS as well as the porosity of the scaffolds are clearly seen. In figure 6b,c, the HeLa cells are shown adhering to the cellulose scaffold, but they also appear to ‘ball-up’ into a spherical morphology and do not spread on the surface of the scaffold. Nevertheless, the HeLa cells appear to proliferate throughout the scaffold during one week of cell culture. This suggests that, even though cellulose as a biomaterial is not the most ideal for the HeLa cells to comfortably spread in a flat fashion, the material can still be considered to be biocompatible and does not result in any detrimental effects repelling away the cells or even causing cell death. Cellulose can thus be considered as a potential biomaterial suitable for various tissue engineering applications.
Figure 6.
Optical microscopy of (a) cellulose scaffold cross-linked with DVS, demonstrating the porous polymeric network (20×), (b) HeLa cells cultured on cellulose (10×), and (c) HeLa cells cultured on cellulose (20×).
(c). Cell culture on chitosan hydrogels
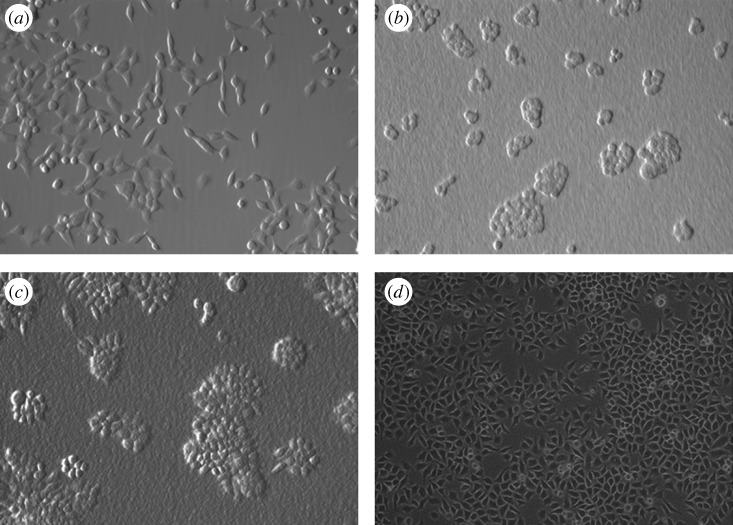
Optical microscopy images showing HeLa cells cultured on Ultrasan are shown in figure 7. Two different Ultrasans were used: one with 91 per cent DDA and the other with 81 per cent DDA. The association between HeLa cells and both the 91 per cent and the 81 per cent DDA Ultrasans appears to be strong, but the cells appear to cluster together (figure 7b,c) and the cell morphology appears to be somewhat different from the control cells (figure 7a). Unlike the control group, where the HeLa cells proliferated evenly throughout the polystyrene surface, the HeLa cells on Ultrasan appear to spread out beginning from selective regions on the scaffold and then proliferate outwards.
Figure 7.
Optical microscopy images of HeLa cells cultured on (a) polystyrene, 2 days (20×), (b) Ultrasan, 91% DDA, 2 days (20×), (c) Ultrasan, 81% DDA, 2 days (20×), and (d) non-Ultrasan chitosan, 75% DDA, 3 days (10×).
In contrast to Ultrasan, the other chitosan hydrogels appear to show even more improved cell attachment and proliferation characteristics, as shown in figure 7d. Clustering of HeLa cells was not observed in these images and the morphology of the cells also appeared to be more similar to the control cells. The cells also proliferated and spread out at a much faster rate compared with what was observed in the case of the Ultrasan samples. This observation indicated that the interactions between the HeLa cells and the chitosan polymer were optimal. Chitosan can thus also be considered as a potential biomaterial suitable for various tissue engineering applications.
(d). Cell culture on alginate hydrogels
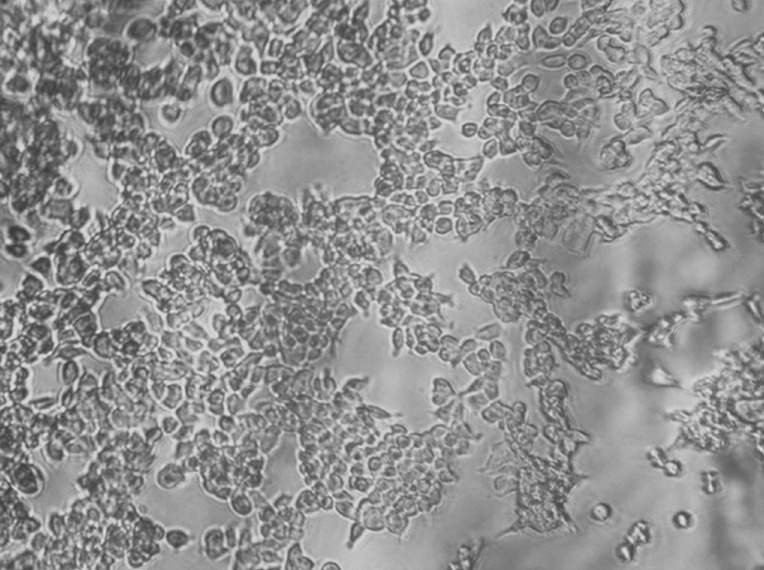
An optical microscopy image showing HeLa cells cultured on alginate is shown in figure 8. Similar results were observed for the alginate hydrogels, where the HeLa cells were seen to attach onto the alginate. The cells proliferated and spread well on the alginate hydrogels, and the morphology of the cells resembled the HeLa cells cultured on polystyrene plastic. Alginate can thus also be considered as a potential biomaterial suitable for various tissue engineering applications.
Figure 8.
Optical microscopy image of HeLa cells cultured on alginate-based scaffold (10×).
4. Conclusions
Naturally derived polymers composed of cellulose, chitosan, alginate and agarose, as well as composites of these polymers, were examined in this paper. The synthesis protocols of single-component scaffolds of pure cellulose, pure chitosan and pure alginate as well as composite scaffolds of cellulose–alginate, cellulose–agarose, cellulose–chitosan, chitosan–alginate and chitosan–agarose were described and discussed. The interactions between HeLa cells and the various different scaffolds were also assessed. The results indicated that interconnected porous networks within the polymeric scaffolds could be induced using the lyophilization technique. It was also demonstrated that HeLa cells attach and proliferate well on the scaffolds. These natural polymer-based scaffolds can therefore be used as potential and suitable biomaterials for various tissue engineering applications.
Acknowledgements
The authors would like to acknowledge the following grants for providing the monetary support of this research: the Pittsburgh Tissue Engineering Initiative, NSF NIRT-CTS-0210238, NIH-NIBIB 1R01EB002706-01 and NIH-NIDCR 5R01DE016123. The authors would also like to thank Marc Rubin, formerly from the Center for Biologic Imaging (University of Pittsburgh), for help with the capture of some of the SEM images reported in this paper. P.N.K. also acknowledges the partial support of the Edward R. Weidlein Chair Professorship funds for supporting this research. The authors also acknowledge NSF-ERC funds for partial support of this research.
Footnotes
One contribution of 14 to a Theme Issue ‘Advanced processing of biomaterials’.
References
- Ahsan T., Bellamkonda R., Nerem R. M.2008Tissue engineering and regenerative medicine: advancing toward clinical therapies Translational approaches in tissue engineering and regenerative medicine Mao J. J., Vunjak-Novakovic A. G. Mikos, Atala A.3–16Norwood, MA: Artech House [Google Scholar]
- Anbergen U., Oppermann W. Elasticity and swelling behavior of chemically crosslinked cellulose ethers in aqueous systems. Polymer. 1990;31:1854–1858. doi: 10.1016/0032-3861(90)90006-K. ( ) [DOI] [Google Scholar]
- Aufderheide A. C., Athanasiou K. A. Comparison of scaffolds and culture conditions for tissue engineering of the knee meniscus. Tissue Eng. 2005;11:1095–1104. doi: 10.1089/ten.2005.11.1095. ( ) [DOI] [PubMed] [Google Scholar]
- Balgude A. P., Yu X., Szymanski A., Bellamkonda R. V. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/S0142-9612(00)00350-1. ( ) [DOI] [PubMed] [Google Scholar]
- Bonfiglio M., Jeter W. S. Immunological responses to bone. Clin. Orthop. Relat. Res. 1972;87:19–27. [PubMed] [Google Scholar]
- Burg K. J. L., Porter S., Kellam J. F. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–2359. doi: 10.1016/S0142-9612(00)00102-2. ( ) [DOI] [PubMed] [Google Scholar]
- Capitani D., Del Nobile M. A., Mensitieri G., Sannino A., Segre A. L. 13C solid-state NMR determination of cross-linking degree in superabsorbing cellulose-based networks. Macromolecules. 2000;33:430–437. doi: 10.1021/ma9914117. ( ) [DOI] [Google Scholar]
- Di Martino A., Sittinger M., Risbud M. V. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;30:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. ( ) [DOI] [PubMed] [Google Scholar]
- Drury J. L., Mooney D. J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. ( ) [DOI] [PubMed] [Google Scholar]
- Ehrenfreund-Kleinman T., Golenser J., Domb A. J. Polysaccharide scaffolds for tissue engineering. In: Ma P. X., Elisseeff J., editors. Scaffolding in tissue engineering. Boca Raton, FL: Taylor & Francis; 2006. pp. 27–44. [Google Scholar]
- Entcheva E., Bien H., Yin L., Chung C.-Y., Farrell M., Kostov Y. Functional cardiac cell constructs on cellulose-base scaffolding. Biomaterials. 2004;25:5753–5762. doi: 10.1016/j.biomaterials.2004.01.024. ( ) [DOI] [PubMed] [Google Scholar]
- Esposito F. A., Del Nobile M. A., Mensitieri G., Nicolais L. Water sorption in cellulose-based hydrogels. J. Appl. Polym. Sci. 1996;60:2403–2407. doi: 10.1002/(SICI)1097-4628(19960627)60:13<2403::AID-APP12>3.0.CO;2-5. ( ) [DOI] [Google Scholar]
- Esposito A., Sannino A., Cozzolino A., Quintiliano S. N., Lamberti M., Ambrosio L., Nicolais L. Response of intestinal cells and macrophases to an orally administered cellulose-PEG based polymer as a potential treatment for intractable edemas. Biomaterials. 2005;26:4101–4110. doi: 10.1016/j.biomaterials.2004.10.023. ( ) [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S., Cohen S., Ziskind A., Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol. Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. ( ) [DOI] [PubMed] [Google Scholar]
- Glicklis R., Shapiro L., Agbaria R., Merchuk J. C., Cohen S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol. Bioeng. 2000;67:344–353. doi: 10.1002/(SICI)1097-0290(20000205)67:3<344::AID-BIT11>3.0.CO;2-2. ( ) [DOI] [PubMed] [Google Scholar]
- Guo J. H., Skinner G. W., Harcum W. W., Barnum P. E. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharm. Sci. Technol. Today. 1998;1:254–261. doi: 10.1016/S1461-5347(98)00072-8. ( ) [DOI] [Google Scholar]
- Gutowska A., Jeong B., Jasionowski M. Injectable gels for tissue engineering. Anat. Rec. 2001;263:342–349. doi: 10.1002/ar.1115. ( ) [DOI] [PubMed] [Google Scholar]
- Hejazi R., Amiji M. Chitosan-based gastrointestinal delivery systems. J. Control. Release. 2003;89:151–165. doi: 10.1016/S0168-3659(03)00126-3. ( ) [DOI] [PubMed] [Google Scholar]
- Helenius G., Backdahl H., Bodin A., Nannmark U., Gatenholm P., Risberg B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A. 2006;76:431–438. doi: 10.1002/jbm.a.30570. ( ) [DOI] [PubMed] [Google Scholar]
- Higashi T., Nagamori E., Sone T., Matsunaga S., Fukui K. A novel transfection method for mammalian cells using calcium alginate microbeads. J. Biosci. Bioeng. 2004;97:191–195. doi: 10.1016/S1389-1723(04)70189-9. (http://www.ncbi.nlm.nih.gov/pubmed/16233613. ) [DOI] [PubMed] [Google Scholar]
- Hu J. C., Athanasiou K. A. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials. 2005;26:2001–2012. doi: 10.1016/j.biomaterials.2004.06.038. ( ) [DOI] [PubMed] [Google Scholar]
- Hutmacher D. W., Goh J. C., Teoh S. H. An introduction to biodegradable materials for tissue engineering applications. Ann. Acad. Med. 2001;30:183–191. [PubMed] [Google Scholar]
- Issa M. M., Köping-Höggård M., Artursson P. Chitosan and the mucosal delivery of biotechnology drugs. Drug Discov. Today. 2005;2:1–6. doi: 10.1016/j.ddtec.2005.05.008. ( ) [DOI] [PubMed] [Google Scholar]
- Jeong B., Kim S. W., Bae Y. H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002;54:37–51. doi: 10.1016/S0169-409X(01)00242-3. ( ) [DOI] [PubMed] [Google Scholar]
- Kim S. E., Park J. H., Cho Y. W., Chung H., Jeong S. Y., Lee E. B., Kwon I. C. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: implications for cartilage tissue engineering. J. Control. Release. 2003;91:365–374. doi: 10.1016/S0168-3659(03)00274-8. ( ) [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Kwon I. C., Kim Y. H., Jo W. H., Jeong S. Y. Preparation of chitosan self-aggregates as a gene delivery system. J. Control. Release. 1998;51:213–220. doi: 10.1016/S0168-3659(97)00173-9. ( ) [DOI] [PubMed] [Google Scholar]
- Li J., Xu Z. Physical characterization of a chitosan-based hydrogel delivery system. J. Pharm. Sci. 2002;91:1669–1677. doi: 10.1002/jps.10157. ( ) [DOI] [PubMed] [Google Scholar]
- Li Z., Ramay H. R., Hauch K. D., Xiao D., Zhang M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. ( ) [DOI] [PubMed] [Google Scholar]
- Lin H.-R., Yeh Y.-J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004;71:52–65. doi: 10.1002/jbm.b.30065. ( ) [DOI] [PubMed] [Google Scholar]
- Liu X., Ma P. X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004;32:477–486. doi: 10.1023/B:ABME.0000017544.36001.8e. ( ) [DOI] [PubMed] [Google Scholar]
- Ma P. X. Alginate for tissue engineering. In: Ma P. X., Elisseeff J., editors. Scaffolding in tissue engineering. Boca Raton, FL: Taylor & Francis; 2006. pp. 13–25. [Google Scholar]
- Miralles G., et al. Sodium alginate sponges with or without sodium hyaluronate: in vitro engineering of cartilage. J. Biomed. Mater. Res. A. 2001;57:268–278. doi: 10.1002/1097-4636(200111)57:2<268::AID-JBM1167>3.0.CO;2-L. ( ) [DOI] [PubMed] [Google Scholar]
- Molinaro G., Leroux J.-C., Damas J., Adam A. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23:2717–2722. doi: 10.1016/S0142-9612(02)00004-2. ( ) [DOI] [PubMed] [Google Scholar]
- Perets A., Baruch Y., Weisbuch F., Shoshany G., Neufeld G., Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A. 2003;65:489–497. doi: 10.1002/jbm.a.10542. ( ) [DOI] [PubMed] [Google Scholar]
- Ratner B. D., Bryant S. J. Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. ( ) [DOI] [PubMed] [Google Scholar]
- Roy K., Mao H. Q., Huang S. K., Leong K. W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999;5:387–391. doi: 10.1038/7385. ( ) [DOI] [PubMed] [Google Scholar]
- Ruel-Gariepy E., Chenite A., Chaput C., Guirguis S., Leroux J.-C. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000;203:89–98. doi: 10.1016/S0378-5173(00)00428-2. ( ) [DOI] [PubMed] [Google Scholar]
- Sannino A., Esposito A., De Rosa A., Cozzolino A., Ambrosio L., Nicolais L. Biomedical application of a superabsorbent hydrogel for body water elimination in the treatment of edemas. J. Biomed. Mater. Res. A. 2003;67:1016–1024. doi: 10.1002/jbm.a.10149. ( ) [DOI] [PubMed] [Google Scholar]
- Sebert P., Bourny E., Rollet M. Gamma irradiation of carboxymethylcellulose: technological and pharmaceutical aspects. Int. J. Pharm. 1994;106:103–108. doi: 10.1016/0378-5173(94)90307-7. ( ) [DOI] [Google Scholar]
- Sivakumar M., Rao K. P. Preparation, characterization, and in vitro release of gentamicin from coralline hydroxyapatite–alginate composite microspheres. J. Biomed. Mater. Res. A. 2003;65:222–228. doi: 10.1002/jbm.a.10495. ( ) [DOI] [PubMed] [Google Scholar]
- Sone T., Nagamori E., Ikeuchi T., Mizukami A., Takakura Y., Kajiyama S., Fukui K. A novel gene delivery system in plants with calcium alginate micro-beads. J. Biosci. Bioeng. 2002;94:87–91. doi: 10.1263/jbb.94.87. ( ) [DOI] [PubMed] [Google Scholar]
- Suh J. -K F., Matthew H. W. T. Applications of chitosan-based polysaccharide biomaterials in cartilage tissue engineering. Biomaterials. 2000;21:2589–2598. doi: 10.1016/S0142-9612(00)00126-5. ( ) [DOI] [PubMed] [Google Scholar]
- Svensson A., Nicklasson E., Harrah T., Panilaitis B., Kaplan D. L., Brittberg M., Gatenholm P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials. 2005;26:419–431. doi: 10.1016/j.biomaterials.2004.02.049. ( ) [DOI] [PubMed] [Google Scholar]
- Varghese S., Elisseeff J. H. Hydrogels for musculoskeletal tissue engineering. In: Werner C., editor. Polymers for regenerative medicine. Vol. 203. Berlin, Germany: Springer; 2006. ( ) [DOI] [Google Scholar]
- Wei G., Ma P. X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. ( ) [DOI] [PubMed] [Google Scholar]
- Xu X. L., Lou J., Tang T., Ng K. W., Zhang J., Yu C., Dai K. Evaluation of different scaffolds for BMP-2 genetic orthopedic tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005;75:289–303. doi: 10.1002/jbm.b.30299. ( ) [DOI] [PubMed] [Google Scholar]
- Yang S., Leong K. F., Du Z., Chua C.-K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. ( ) [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. A. 2001;55:304–312. doi: 10.1002/1097-4636(20010605)55:3<304::AID-JBM1018>3.0.CO;2-J. ( ) [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. A. 2002;62:378–386. doi: 10.1002/jbm.10312. ( ) [DOI] [PubMed] [Google Scholar]