Abstract
Dietary methionine restriction (MR) is a mimetic of chronic dietary restriction (DR) in the sense that MR increases rodent longevity, but without food restriction. We report here that MR also persistently increases total energy expenditure (EE) and limits fat deposition despite increasing weight-specific food consumption. In Fischer 344 (F344) rats consuming control or MR diets for 3, 9, and 20 mo, mean EE was 1.5-fold higher in MR vs. control rats, primarily due to higher EE during the night at all ages. The day-to-night transition produced a twofold higher heat increment of feeding (3.0°C vs. 1.5°C) in MR vs. controls and an exaggerated increase in respiratory quotient (RQ) to values greater than 1, indicative of the interconversion of glucose to lipid by de novo lipogenesis. The simultaneous inhibition of glucose utilization and shift to fat oxidation during the day was also more complete in MR (RQ ∼0.75) vs. controls (RQ ∼0.85). Dietary MR produced a rapid and persistent increase in uncoupling protein 1 expression in brown (BAT) and white adipose tissue (WAT) in conjunction with decreased leptin and increased adiponectin levels in serum, suggesting that remodeling of the metabolic and endocrine function of adipose tissue may have an important role in the overall increase in EE. We conclude that the hyperphagic response to dietary MR is matched to a coordinated increase in uncoupled respiration, suggesting the engagement of a nutrient-sensing mechanism, which compensates for limited methionine through integrated effects on energy homeostasis.
Keywords: energy expenditure, metabolic efficiency, oxidative metabolism, futile cycles, adipose tissue, dietary restriction
dietary methionine restriction (MR) extends lifespan by 30–35% in rats (28, 31) and mice (27) by delaying all causes of death. The increase in lifespan is accompanied by a reduction in adiposity that occurs despite a paradoxical increase in weight-specific food consumption (25, 28, 46). Pair-feeding studies comparing rats fed the control diet to the amount of MR diet consumed by the MR group clearly show that dietary MR decreases metabolic efficiency (25, 46), but the underlying basis for the metabolic responses to dietary MR remains poorly understood. Short- (12 wk) and long-term (80 wk) consumption of the MR diet after weaning also reduced circulating triglyceride, insulin, and leptin while increasing plasma adiponectin (25, 29). Collectively, work to date makes a compelling case that limitation of fat deposition by dietary MR is associated with preservation of insulin sensitivity and significant improvements in metabolic markers of lipid metabolism. Using the tools of metabolic phenotyping to examine energy homeostasis and peripheral substrate utilization, we found that dietary MR produced a significant long-term increase in EE that was temporally linked to exaggerated thermogenic responses to feeding and modest increases in resting EE. These physiological responses to MR limited fat deposition and were associated with significant changes in the metabolic and endocrine function of brown and white adipose tissue. MR effectively increased EE and limited fat deposition when the dietary regimen was provided to young growing animals, to mature animals, or to obesity-prone Osborne-Mendel rats in a high-fat, calorically dense formulation. Collectively, these studies support the concept that dietary MR limits fat deposition by decreasing metabolic efficiency, with the largest effect occurring at night during periods when glucose utilization is being used to support an increase in de novo lipogenesis.
MATERIALS AND METHODS
Animals and diets.
All experiments were reviewed and approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee on the basis of guidelines established by the National Research Council, the Animal Welfare Act, and the Public Health Service Policy on the humane care and use of laboratory animals. Four experiments were conducted using male F344 rats obtained from Harlan (Indianapolis, IN) immediately after weaning at 4 wk of age (experiments 1–3) or at 5 mo of age (experiment 4). A fifth study was conducted with 4-wk-old male Osborne-Mendel rats obtained from a breeding colony maintained at the Pennington Center. In experiments 1–3, the rats were fed Purina rodent diet (#5001) until 32 days of age, then singly housed in shoebox cages with corncob bedding and randomly assigned to one of three dietary treatment groups. Using the experimental feeding paradigm described previously (25, 28, 31), rats in the control group were provided a purified control diet containing 0.86% methionine, while rats in the MR group were provided the same diet with methionine restricted to 0.17%. Both diets were provided ad libitum. The diets were formulated as extruded pellets and their composition is provided in Table 1. A third group (DR) was fed the control diet but with their intake restricted to 60% of the average daily intake of the ad libitum-fed control group, determined in preliminary studies with mature F344 rats to be 15 g/day. On the basis of an average intake of 15 g/day for the control group, the DR group was provided 9 g/day for the duration of each experiment. The energy content of both control and MR diets (Dyets, Bethlehem, PA) was 15.96 kJ/g, with 18.9% of energy coming from fat (corn oil), 64.9% carbohydrate, and 14.8% from a custom mixture of L-amino acids (Table 1). The amino acid content of the diet on a weight basis was 14.1%. Water was provided ad libitum to all treatment groups, room temperature was maintained at 22–23°C, and lights were on 12 h/day from 7 AM to 7 PM. In experiment 4, 5-mo-old rats were singly housed as above and fed the control diet for 1 mo prior to random assignment to receive either the control or MR diet ad libitum. In experiment 5, weanling male Osborne-Mendel rats were singly housed and fed the control diet until 32 days of age, then randomly assigned to receive a high-fat diet containing either 0.86% or 0.17% methionine ad libitum. The energy content of both control and MR diets was 22.46 kJ/g, with 60% of energy coming from fat (4% from soybean oil and 56% from coconut oil), 28% from carbohydrate, and 10.5% from the amino acid mixture. The health of animals used in these studies was monitored using sentinel animals housed with experimental animals and monitored at regular intervals.
Table 1.
Composition of the methionine-restricted diet
| Ingredient | Concentration in Diet, % | Ingredient | Concentration in Diet, % |
|---|---|---|---|
| l-Arginine | 1.12 | l-Phenylalanine | 1.16 |
| l-Lysine | 1.80 | Glycine | 2.33 |
| l-Histidine | 0.33 | Dextrose | 20.00 |
| l-Leucine | 1.11 | Dyetrose | 5.00 |
| l-Isoleucine | 0.82 | Corn starch | 43.25 |
| l-Valine | 0.82 | Cellulose fiber | 5.00 |
| l-Threonine | 0.82 | Choline bitartrate | 0.20 |
| l-Tryptophan | 0.18 | Vitamin mix - AIN-76A | 1.00 |
| dl-Methionine* | 0.17 | Mineral mix - AIN-76 | 3.50 |
| Glutamic acid† | 3.39 | Corn oil | 8.00 |
Energy content of control and methionine-restricted diets is 3.812 kcal/g.
dl-Methionine concentration of control diet is 0.86%.
l-Glutamic acid concentration of control diet is 2.70%.
Food consumption was measured at specific intervals in each experiment by weighing the food provided at the beginning of the feeding interval and weighing the unconsumed and wasted food 24 or 48 h later between 3 and 7 PM. The corncob bedding was sifted through wire mesh to retrieve and weigh any food pellets removed from the food dispenser but not consumed. Body composition was determined by dissection in experiments 1 and 2, but with procurement of additional instrumentation, by dual-energy X-ray absorptiometry (DEXA, Hologic QDR-1000, Waltham, MA) in experiments 3 and 5, and by NMR spectroscopy (Bruker Minispec, Billerica, MA) in experiment 4 using the calibration standards and operational protocols of the respective manufacturers.
Interscapular BAT and WAT depots were carefully dissected at the end of each experiment, and total RNA was isolated for measurement of uncoupling protein 1 (UCP1), leptin, or adiponectin mRNA, as previously described (45). In experiments 2 and 3, whole cell extracts were also prepared from interscapular BAT for measurement of UCP1 protein expression as previously described (3, 5).
Experiment 1.
Three groups of 18 rats were studied for 8 wk after weaning to compare the short-term effects of dietary MR and 40% DR. Food consumption was monitored at 1, 2, 4, and 8 wk after weaning onto the respective diets, and cohorts of 6 animals from each group were killed after 2, 4, and 8 wk to assess fat deposition among the groups. Blood samples were obtained at 8 wk for assay of plasma insulin, leptin, and adiponectin (25). Energy intake per unit body weight was calculated for each treatment group and week of the study.
Experiment 2.
To assess short-term effects of MR on energy expenditure, three groups (control, MR, and DR) of 8 rats were provided the respective diets for 12 wk beginning at 5 wk of age. After 12 wk on the diets, the rats were transferred to the Small Animal Phenotyping Core Facility at the Pennington Biomedical Research Center for measurement of EE by indirect calorimetry (Oxymax System, Columbus Instruments, Columbus, OH). The rats were acclimated in the metabolic chambers overnight prior to measurement of oxygen consumption (Vo2) and carbon dioxide production (Vco2) at 48-min intervals for 72 h. The Oxymax calorimeter was calibrated once every 24 h. Control and MR rats had free access to food and water during this period, and DR rats were provided their daily food allotment between 4 and 6 PM. At the end of the 3-day period, the rats were removed from the calorimeter, euthanized, and the inguinal, retroperitoneal, epididymal, and interscapular fat pads were carefully dissected and weighed to estimate fat and fat-free content of the carcass. Vo2 is expressed as liters of O2 consumed per hour, while respiratory quotient (RQ) is the ratio of Vco2 produced to Vo2 consumed. EE was calculated as {Vo2 × (3.815 + [1.232 × RQ)] × 4.019 kJ/h} and expressed as kilojoule per hour per kilogram fat-free mass (FFM) (35).
Experiment 3.
To assess chronic effects of MR on body composition, energy expenditure, core body temperature, and voluntary activity, two groups of 16 rats were provided the control or MR diets beginning at 5 wk of age. Body weight (BW) was measured weekly and 48-h food consumption was determined after 6 wk and every other week thereafter for the duration of the study. After 6 and 12 mo, core body temperature was measured at the midpoint of the light and dark cycles over a 3-day period using a rat rectal thermocouple probe (MLT1403, AD Instruments, Colorado Springs, CO) using procedures recommended by the manufacturer. At 9 and 20 mo, cohorts of 8 rats from each group were transferred to the Oxymax system for measurement of energy expenditure by indirect calorimetry as described above. The rats were acclimated in the metabolic chambers overnight prior to measurement of Vo2 and Vco2 at 30-or 48-min intervals for 96 h. At the end of the 4-day period, the rats were removed from the calorimeter and weighed, and body composition was determined by DEXA. EE was calculated as before and expressed per unit FFM (35). Lastly, voluntary activity in their home cages was measured for rats in each group over three distinct 4-day periods using the Opto-Varimax system (Columbus Instruments). Beam breaks by each rat were measured during the period when lights were off (7 PM–7 AM) and on (7 AM–7 PM), and voluntary activity was expressed as beam breaks per hour during each period.
Experiment 4.
To assess the effects of MR on body composition and energy expenditure in rats after attainment of physical maturity, two groups of 6-mo-old rats (16/group) were provided the control or MR diet for 6 mo thereafter. BW, body composition, and 48-h food consumption were determined weekly for the duration of the study. After 3 and 6 mo, cohorts of 8 rats per group were transferred to the Oxymax system for measurement of energy expenditure as described above. The rats were acclimated in the metabolic chambers overnight prior to measurement of oxygen consumption (Vo2) and carbon dioxide production (Vco2) at 48-min intervals for 4 days. Thereafter, the rats were weighed, and body composition was determined by NMR.
Experiment 5.
To assess the effects of MR in the context of increased caloric density, two groups of male, 5-wk-old obesity-prone Osborne-Mendel rats (6–9/group) were provided the control or MR diets formulated to contain 60% of energy as fat as described above. The high-fat control and MR diets were provided for 6 mo. BW and 48-h food intake were determined weekly, and body composition was determined by DEXA after 8, 16, and 26 wk.
Methods of analysis.
Body weight, food consumption, body composition, core body temperature, UCP1 mRNA, UCP1 protein, and voluntary activity were compared using ANOVA followed by post hoc testing of treatment differences at each time point. For experiments 2–4, the calorimetric data (RQ & EE) were compared using two approaches. First, to assess dietary effects spanning both phases of the diurnal cycle, the average RQ and EE over the period of measurement were analyzed by one-way ANOVA. Post hoc testing of group means within each experiment and age was made using the Bonferroni correction and the pooled error term from the ANOVA to calculate standard errors. To test for diet effects within the light and dark phases of the diurnal cycle, the average RQs and EEs during the light and dark cycles at each age were compared by two-way ANOVA using diet and diurnal phase as main effects. The diet × diurnal phase interaction was tested using residual variance (animal within diet × phase) as the error term, and post hoc testing of group means within each experiment and diet was also made using the Bonferroni correction and pooled error term to calculate standard errors. Protection against type I errors was set at 5% (α=0.05).
RESULTS
Experiment 1.
Previous studies indicate that chronic dietary MR reduces fat deposition while increasing weight-specific food consumption (25, 28). Experiment 1 was undertaken to examine the acute responses to dietary MR. Energy intake adjusted for BW did not differ between MR (155 ± 5 kJ day−1 100 g BW−1) and control rats (143 ± 4 kJ day−1 100 g BW−1) during the first wk, but by week 2 and thereafter (Fig. 1A), food intake in the MR group was 30% higher (P < 0.01). By design, energy intake in the DR group was limited to 144 kJ per day, so the change in BW-adjusted energy intake in the DR group reflects the increase in BW over the 8-wk study (Fig. 1A).
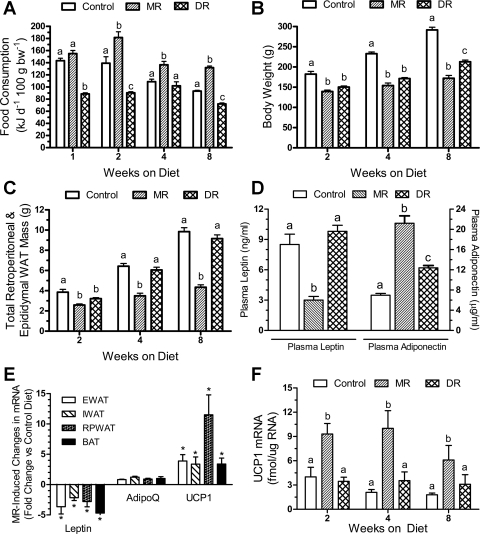
Fig. 1.
Postweaning responses of male Fischer 344 rats to dietary methionine restriction (MR) or 40% dietary restriction (DR). At 32 days of age, rats were randomly assigned to groups that would receive a control diet containing 0.86% methionine (control group), a diet with methionine restricted to 0.17% (MR), or a DR group that would receive the control diet at 60% of the consumption of the control group. Energy intake (A), body weight (B), relative adiposity (white adipose tissue, WAT) (C), and brown adipose tissue (BAT) UCP1 mRNA (F) were measured in separate cohorts of 8 rats per treatment per time point after 2, 4, and 8 wk on the respective diets. Plasma leptin and adiponectin (D) were measured in samples from the 8-wk time point, as were the mRNA levels for leptin, adiponectin, and UCP1 in the WAT and BAT tissues collected after 8 wk (E). EWAT, epididymal white adipose tissue; IWAT, inguinal white adipose tissue; RPWAT, retroperitoneal white adipose tissue. Response variables were analyzed by ANOVA. a,b,cMeans at each time point with letters that differ denote P < 0.05. E: *P < 0.05, significant difference for each gene and tissue compared with control group.
In experiment 1, retroperitoneal and epididymal fat pad weights and BW-adjusted fat pad weights were used as surrogate measures of adiposity. The rapid effect of dietary MR on food consumption (Fig. 1A) was paralleled by a significant decrease (P < 0.01) in BW and adiposity of MR rats relative to the control group after 2 wk (Fig. 1, B and C). However, relative adiposity continued to increase between 4 and 8 wk in the control and DR groups and surprisingly, was comparable between both groups (Fig. 1C). Thus, BW accretion was slowed in the DR group (Fig. 1B), but fat deposition was preserved at the expense of overall growth (Fig. 1C). This finding is supported by the comparable plasma leptin levels in control and DR groups after 8 wk (Fig. 1D). In contrast, plasma leptin was ∼3-fold lower and adiponectin was 3-fold higher in the MR group than controls (Fig. 1D). To test for a transcriptional mechanism, leptin and adiponectin mRNA levels were expressed relative to the mean expression level of the control group for each depot. Dietary MR decreased leptin mRNA by 2- to 5-fold across all depots (Fig. 1E), but the MR-induced increase in plasma adiponectin (Fig. 1D) was not matched by any change in adiponectin mRNA among any depot (Fig. 1E). A reduction of plasma leptin was expected with the MR-induced reduction of fat mass (∼25%), but the magnitude of the respective changes in leptin and adiponectin argues that the effects were not merely secondary to reduced adipose tissue. In BAT, UCP1 mRNA levels did not differ between control and DR rats after 2, 4, or 8 wk (Fig. 1F), but dietary MR increased UCP1 mRNA by ∼3-fold at all 3 times relative to controls (Fig. 1F). Dietary MR also increased UCP1 mRNA by ∼4-fold in epididymal and inguinal WAT and by >10-fold in the retroperitoneal depot (Fig. 1E).
Experiment 2.
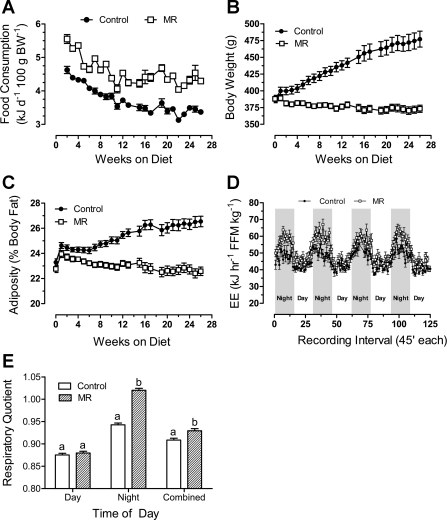
To examine the effects of short-term MR (3 mo) on EE and substrate utilization, indirect calorimetry was used to measure O2 consumption and CO2 production during the postweaning growth phase. EE changed in phase with the light:dark cycle and was highest at night when rats were awake and eating, and lowest during the day when rats normally sleep (Fig. 2A). EE in the MR group was 70–80% higher (P < 0.01) than the other groups at night, but only ∼25% higher during the day when reaching its diurnal nadir (Fig. 2A).
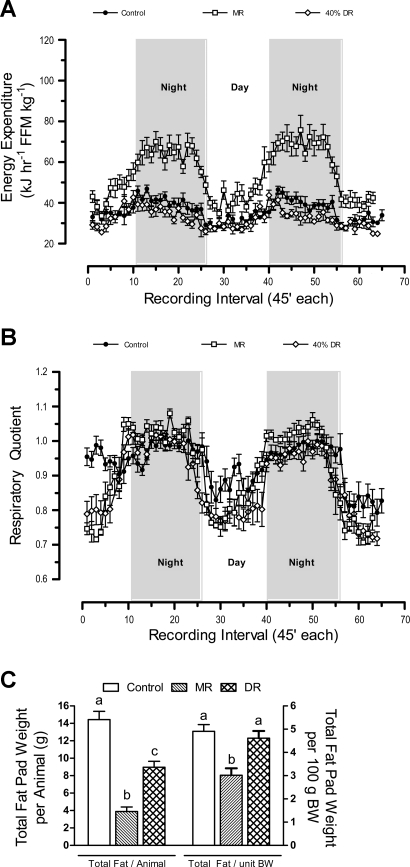
Fig. 2.
Energy expenditure (EE; A), respiratory quotient (B), and fat deposition (C) in male Fischer 344 rats after dietary methionine restriction (MR) or 40% dietary restriction (DR) for 3 mo. At 32 days of age, 8 rats per group were provided a control diet containing 0.86% methionine (control group), a diet with methionine restricted to 0.17% (MR), or the control diet restricted to 60% of the consumption of the control group. The rats were adapted to the calorimetry chambers for 24 h prior to measurement of O2 consumption and CO2 production at 45-min intervals for the following 3 days. Thereafter, fat pads were dissected and weighed, and EE and respiratory quotient were calculated as described in materials and methods. Average EE was calculated for each animal in each group for the period when lights were on (7 AM–7 PM), when lights were off (7 PM–7 AM), and averaged over both periods to assess total daily EE. FFM, fat-free mass; BW, body weight. These 3 variables and total fat pad weights were compared by ANOVA. a,b,cMeans with letters that differ denote P < 0.05.
The respiratory quotient (RQ) provides a real-time index of substrate utilization during the metabolic cycle and is based on the molar ratios of O2 consumed and CO2 produced during the oxidation of glucose (1.00), lipid (0.70), and protein (0.80) (6, 9, 33). RQs typically range toward 1 during the switch to glucose utilization in the fed state and toward 0.7 during the switch to fat utilization during fasting. Fig. 2B shows the expected diurnal fluctuations in RQ, approaching 1 at night when rats consume 75–85% of their daily intake (8, 23). However, the average night-time RQ in the MR group exceeded 1 (Fig. 2B), which only occurs when glucose utilization is used to support de novo lipogenesis (6, 33). During the day-time postabsorptive state, RQs in the MR and DR groups dropped to ∼0.75 (Fig. 2B), indicating a near-complete shift to lipid utilization as oxidative fuel. Viewed together, Fig. 2, A and B indicate that the high rate of EE at night in MR rats is linked to a high rate of glucose utilization and interconversion to lipid via de novo lipogenesis. In addition, the range of day-to-night excursions in RQ were larger in the MR group than in either of the other groups, consistent with enhanced metabolic flexibility in the MR group (Fig. 2B).
The higher EE in the MR group at 3 mo was translated into lower dissectible white adipose tissue (WAT) relative to the control group (Fig. 2C). Total dissectible WAT in DR rats was intermediate between control and MR groups, but when expressed per unit of BW, their adiposity did not differ from controls (Fig. 2C). These data show that the limitation of growth and fat deposition by DR was proportional to the restriction of energy intake. In contrast, MR rats deposited 40% less fat per unit of BW than either control or DR rats (Fig. 2C), while their energy intake (131 ± 3 kJ·day−1·100 g BW−1; n = 8) was significantly higher (P < 0.01) than the control group (96 ± 6 kJ·day−1·100 g BW−1; n = 8). Collectively, these data show that the metabolic inefficiency induced by the MR diet compensated for the hyperphagia to an extent sufficient to limit fat deposition.
Experiment 3: Growth and Fat Deposition.
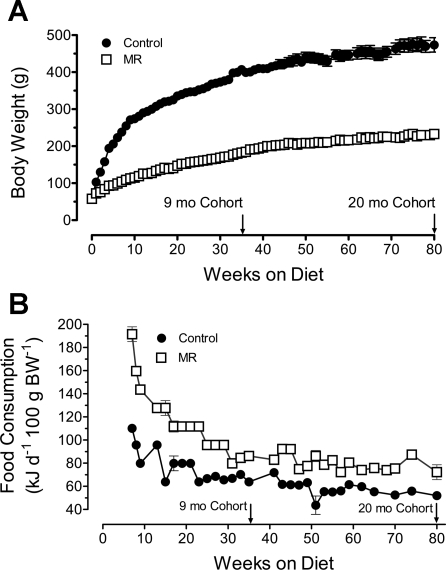
Experiments 1 and 2 showed that dietary MR affected energy homeostasis during the rapid postweaning phase of growth. To examine the duration of these effects, rats were evaluated after consuming the MR diet for 9 and 20 mo. Fig. 3A shows that MR produced an immediate decrease in growth rate that was maintained for the duration of the study. For example, the BW of the MR group (185 ± 4.2 g, n = 16) was approximately half that of the control group (399 ± 11 g, n = 16) at 36 wk and 80 wk (MR: 233 ± 8.1 g, n = 8; controls: 474 ± 19.4 g, n = 8). Energy intake was relatively constant between 7 and 80 wk in control (251 kJ ± 4 kJ/day, n = 31) and MR rats (164 ± 3 kJ/d, n = 31), but when expressed relative to BW, energy intake per 100 g BW was significantly higher (P < 0.01) in MR rats compared with controls at all time points from weaning to 80 wk (Fig. 3B). This illustrates that dietary MR induced a chronic state of metabolic inefficiency that translated into higher maintenance requirements of the MR group.
Fig. 3.
Postweaning growth (A) and energy intake (B) of male Fischer 344 rats provided a control or methionine-restricted diet. At 32 days of age, rats were randomly assigned to receive a control diet containing 0.86% methionine (control group) or a diet with methionine restricted to 0.17% (MR). The diets were provided ad libitum, and cohorts (8–10 rats/cohort) of each group consumed their respective diets for 9 or 20 mo after weaning. Body weights were determined weekly, and food consumption was measured every other week.
The MR diet also reduced long-term fat accretion by 30%, with adiposity of MR rats limited to ∼20%, compared with 27.4 ± 1.3% in the controls. This is similar to the 26% and 34% reduction in adiposity produced by dietary MR after 2 (Fig. 1D) and 3 mo (Fig. 2C). It should be noted that relative adiposity was assessed by fat pad mass in experiments 1 and 2, while DEXA was used in experiment 3. Although the approaches are fundamentally different and used at different ages, the assessment of relative adiposity between groups was comparable for both methods. And although the measurements of adiposity by DEXA at 9 and 20 mo are understandably more accurate, the data from both methods support a common conclusion that MR limited fat deposition at all ages.
Indirect calorimetry.
Indirect calorimetry was used to examine the chronic effects of dietary MR on EE and substrate utilization as a basis for understanding the day-to-night effects of MR on growth and nutrient partitioning. At both 9 and 20 mo, the daily excursions of EE (Fig. 4, A and C) and RQ (Fig. 4, B and D) followed the expected diurnal pattern, but there were significant age-dependent changes in both pattern and intensity of respiration among the groups. EE was highest at night and lowest during the day (Figs. 4, A and C). The night-time increase in EE was significantly higher (P < 0.01) in the MR group compared with controls at both 9 mo (Fig. 4A, Table 2) and 20 mo (Fig. 4C, Table 2). In addition, day-time EE was also 90% higher in the MR group than controls at the 9-mo time point (Table 2), but the difference decreased to 25% at 20 mo (Table 2). Viewed alongside results from 3 mo, total EE was relatively constant in controls between 3 and 20 mo and significantly lower than the MR group at all three time points (Fig. 2A, Table 2).
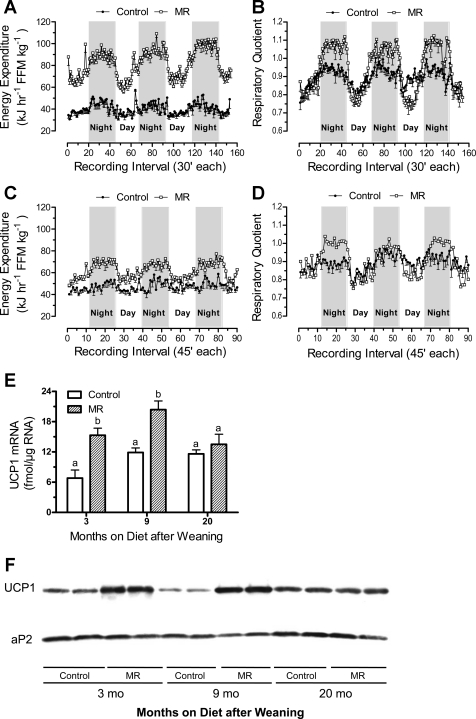
Fig. 4.
Diurnal changes in energy expenditure, respiratory quotient, and UCP1 expression in BAT from male Fischer 344 rats consuming a control or methionine-restricted diet (MR) for 9 (4, A and B) or 20 mo (4, C and D). At 32 days of age, rats were randomly assigned to receive a control diet containing 0.86% methionine (control group) or a diet with methionine restricted to 0.17% (MR). The diets were provided ad libitum, and cohorts (8–10 rats/cohort) of each group consumed their respective diets for 9 or 20 mo after weaning. After 9 and 20 mo, rats from the respective control and MR cohorts were adapted to the calorimetry chambers for 24 h prior to measurement of O2 consumption and CO2 production at 30-min intervals for the following 3 days. Thereafter, body composition of each rat was determined by DEXA scanning as described in materials and methods and used to express EE per unit of fat-free mass. Average EE was calculated for each animal in each group for the period when lights were on (7 AM–7 PM), when lights were off (7 PM–7 AM), and averaged over both periods to assess total daily EE. The means were compared by ANOVA at each age (Table 1). UCP1 mRNA (E) and protein expression (F) were measured in BAT after 3 (experiment 2), 9, and 20 mo on the respective diets. The responses were compared by ANOVA. a,bMeans at each time point with letters that differ denote P < 0.05.
Table 2.
Diurnal variation in energy expenditure in rats fed a control (0.86% methionine) or methionine-restricted diet (0.17% methionine) ad libitum for 9 or 20 mo
| Average EE (kJ h−1 FFM kg−1 ± SE) |
||||
|---|---|---|---|---|
| Dietary Treatment | Time on Diet | Day (7 AM–7 PM) | Night (7 PM–7 AM) | Day and Night |
| Control | 9 mo | 37.4 ± 0.35a | 43.5 ± 0.45a | 40.4 ± 0.30a |
| MR | 72.1 ± 0.54b | 93.7 ± 0.53b | 82.5 ± 0.38b | |
| Control | 20 mo | 45.5 ± 0.40a | 49.9 ± 0.61a | 47.2 ± 0.35a |
| MR | 56.9 ± 0.52b | 69.1 ± 0.64b | 62.6 ± 0.47b | |
Representative 32 day-old male Fischer 344 rats were randomly assigned to receive a control diet containing 0.86% methionine or a diet with methionine restricted to 0.17% (MR). The diets were provided ad libitum, and cohorts of each group consumed their respective diets for 9 and 20 mo. At the 9- and 20-mo time points, body composition of each animal was measured by DEXA and energy expenditure (kJ h−1 FFM kg−1, where FFM is fat-free mass) was measured continuously for 4 days by indirect calorimetry as described in materials and methods. Average energy expenditure (EE) was calculated for each animal in each group (n = 8) for the period when lights were on (7 AM–7 PM), for the period when lights were off (7 PM–7 AM), and averaged over both periods to assess total EE. Mean daytime, nighttime, and total EE were analyzed by one-way ANOVA at each age. a,b Means that have a different letter superscript within each age group differ at P < 0.05.
The diurnal patterns of change in RQ at 9 (Fig. 4B) and 20 mo (Fig. 4D) were also similar to 3 mo but with a slight difference in the MR group at 20 mo compared with 3 and 9 mo. First, RQs consistently exceeded 1 at night in the MR group after 9 and 20 mo (Fig. 4, B and D), but they were most prominent at 9 mo (Fig. 4B), with RQs consistently between 1.05 and 1.1 for most of the dark cycle. In contrast, nighttime RQs in control rats were close to but consistently less than 1 (Fig. 4, B and D). Second, the range of day-to-night excursions in RQ was larger in MR vs. controls at 3, 9, and 20 mo, but the magnitude of the difference was greatest at 9 mo (Figs. 2B and 4, B and D). The largest difference in EE between groups also occurred at 9 mo (Figs. 2A, 4, A and C), with the smallest difference observed after 20 mo.
In vivo thermogenesis, UCP1 expression, and ambulatory activity.
Decreased efficiency of energy utilization for growth in the MR group implies energy wasting through some combination of substrate cycling, uncoupling of fuel oxidation from ATP generation, or increased voluntary activity. To address the latter possibilities, core body temperature was measured during the two phases of the metabolic cycle after 6, 12, and 20 mo, while UCP1 expression was examined after 3, 9, and 20 mo. Ambulatory activity was measured after 20 mo. Day-time core temperatures did not differ between groups at any age and were unchanged between 6 and 20 mo (means = 34.0 ± 0.12°C, n = 8). Night-time core temperatures increased in both groups, and the increase averaged between 0.8 and 1.4°C in controls at both 6 and 12 mo. In contrast, the night-time increase in the MR group was significantly greater than the control group (P < 0.05) (Table 3), averaging 3°C at 6 mo and 2°C at 12 mo. The increase was twofold greater than the response in the Control group at both time points, consistent with the magnitude of difference in EE between groups at these times. However, by 20 mo the night-time increase in core temperature did not differ between groups (Table 3), and this was primarily due to a decrease in the magnitude of the response to MR.
Table 3.
Diurnal variation in core body temperature and ambulatory activity in rats fed a control (0.86% methionine) or methionine-restricted diet (0.17% methionine) ad libitum for 6, 12, or 20 mo
| Core Body Temperature (°C ± SE) |
Ambulatory Activity (Beam Breaks ± SE) |
|||
|---|---|---|---|---|
| Dietary Treatment | Time on Diet | Night (11 PM–1 AM) | Day (7 AM–7 PM) | Night (7 PM–7 AM) |
| Control | 6 mo | 35.4 ± 0.28a | ||
| MR | 37.0 ± 0.25b | |||
| Control | 12 mo | 35.2 ± 0.18a | ||
| MR | 36.0 ± 0.19b | |||
| Control | 20 mo | 36.1 ± 0.19a | 2271 ± 121a | 5500 ± 120a |
| MR | 35.5 ± 0.20a | 1579 ± 67b | 6300 ± 270a | |
Representative 32 day-old male Fischer 344 rats were randomly assigned to receive a control diet containing 0.86% methionine or a diet with methionine restricted to 0.17% (MR). The diets were provided ad libitum, and cohorts of each group consumed their respective diets for 6, 12, or 20 mo. After 6, 12, and 20 mo on the respective diets, core body temperature was measured at 30-min intervals between 1 and 3 PM (day) and between 11 PM and 1 AM (night), as described in materials and methods. Daytime body temperatures averaged 34.0 ± 0.12°C and did not differ among groups or change with age. Mean body temperatures during the night were compared by one-way ANOVA. a,b Means having a different letter superscript within each age differ at P < 0.05. After 20 mo on the respective diets, voluntary ambulatory activity was measured in their home cages for three distinct 4-day periods for rats in each group as described in materials and methods. Voluntary activity was expressed as beam breaks per hour and mean daytime and nighttime beam breaks were analyzed by ANOVA. a,b Means with a different letter superscript within each time of day differ at P < 0.05.
Sympathetic stimulation of BAT increases its thermogenic function by simultaneously increasing UCP1 expression and uncoupling activity of expressed protein. UCP1 mRNA and protein were measured in relation to the increase in EE and core temperature in MR rats and as a marker for increased sympathetic nervous system (SNS) stimulation of BAT. After 3 and 9 mo, UCP1 mRNA and protein were 2-fold higher in BAT of MR rats compared with controls (Fig. 4, E and F). In contrast, UCP1 expression did not differ between controls and the MR group at 20 mo (Fig. 4, E and F). These findings show that dietary MR produces a rapid and persistent increase in UCP1 expression that wanes with age. Lastly, measurement of ambulatory activity of rats in their home cages after 20 mo on the respective diets provided no evidence that the higher EE in MR rats was attributable to increased voluntary activity at night (Table 3). In addition, ambulatory activity of the MR group during the day was significantly lower than that of the control group (Table 3). Together, these findings do not support an increase in physical activity as a basis for the higher EE or core temperature in the fed state in MR vs. control rats, or for the higher day-time EE in MR rats.
Experiment 4. MR limits fat deposition and increases EE in adult rats.
The effects of dietary MR when initiated after physical maturity were assessed in rats beginning at 6 mo of age. Within 2 wk, dietary MR increased weight-adjusted energy intake by 20% (P < 0.01) over controls and maintained a 20–25% higher rate of energy intake for the 6-mo duration of the study (Fig. 5A). The average initial BW of the two groups was 388 ± 4.2 g. The control group grew at a fairly constant rate, reaching 477 ± 11.9 g by study's end (Fig. 5B). In contrast, despite a 20% higher rate of weight-specific energy intake, MR rats did not increase BW during the study, maintaining 96% of their initial weight and ending the study at 373 ± 3.3 g (Fig. 5C). The initial adiposity of the control group was 23 ± 0.3%, which increased to 26.5 ± 0.4% by study's end (Fig. 5C). In contrast, the ending adiposity of the MR group (22.6 ± 0.3%) did not differ from their initial adiposity. The percentage of FFM of the MR group also did not change, indicating that dietary MR preserved both body weight and body composition in the mature rat over a period of 6 mo.
Fig. 5.
Energy intake (A), growth (B), fat deposition (C), and metabolic responses (D and E) of adult Fischer 344 rats to dietary methionine restriction. At 6 mo of age, rats were randomly assigned to receive a control diet containing 0.86% methionine (control group) or a diet with methionine restricted to 0.17% (MR). The diets were provided ad libitum. Food consumption, body weight, and body composition were measured weekly for the subsequent 26 wk. Thereafter, rats were adapted to the calorimetry chambers for 24 h prior to measurement of O2 consumption and CO2 production at 45-min intervals for the following 4 days. Body composition was determined by NMR using a Bruker Minispec and used to express energy expenditure per unit of fat-free mass as described in materials and methods. Average EE and RQ were calculated for each animal in each group for the period when lights were on (7 AM–7 PM), when lights were off (7 PM–7 AM), and averaged over both periods to assess total daily EE. Energy intake, body weight, adiposity, EE, and RQ were compared by one-way ANOVA at each time point as described in materials and methods. a,bLetters that differ denote P < 0.05.
Calorimetric measures after 6 mo show that night-time EE was significantly higher (P < 0.01) in the MR group (60.1 ± 0.4 kj·h−1 kg·FFM−1) than controls (49.6 ± 0.4 kj·h−1·kg FFM−1). As evident from the RQ data, the major difference occurred during the day-to-night shift to glucose utilization in the MR group, while day-time RQ did not differ between groups (Fig. 5E).
BAT UCP1 expression was also examined and dietary MR increased UCP1 mRNA in BAT by approximately twofold (20.1 ± 1.1 fmol mRNA/μg RNA) compared with controls (12.2 ± 1.4 fmol mRNA/μg RNA) after 3 mo. However, after 6 mo UCP1 mRNA levels did not differ between the groups. The lack of any comparable diminution in the effect of dietary MR on EE between 3 and 6 mo suggests that the diet-induced increase in UCP1 expression is not necessary for MR to increase EE.
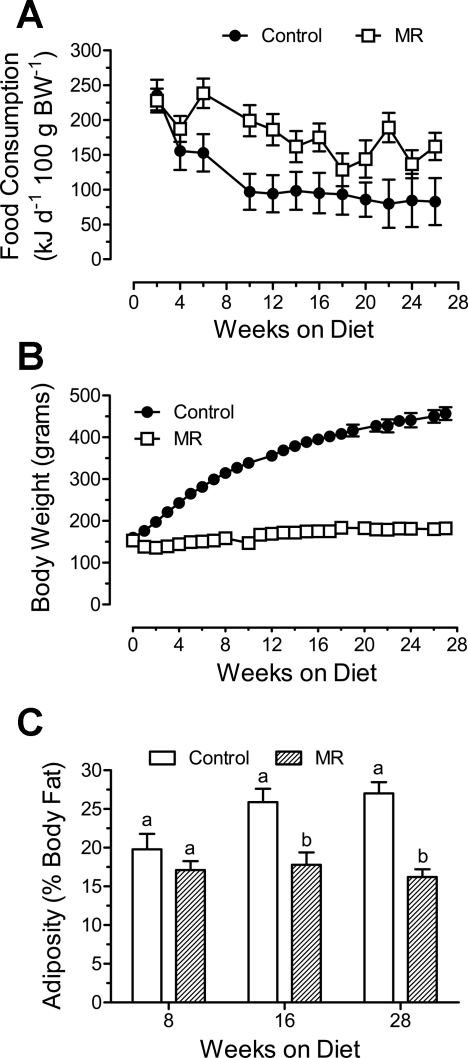
Experiment 5. MR limits fat deposition in obesity-prone Osborne-Mendel rats.
The MR diet provided in experiments 1–4 was a relatively low-fat (10% by weight) formulation with low-caloric density (16.0 kJ/g). In each experiment, the increase in EE was sufficient to compensate for the hyperphagic responses to the MR diet and limit fat accretion. Therefore, the objective of experiment 5 was to determine whether increasing the caloric density of the MR diet to 60 kcal% (22.5 kJ/g) would affect either component of the response in fat-preferring, obesity-prone Osborne-Mendel rats, and whether fat deposition would be limited. Energy intake did not differ between control and MR rats for the first 4 wk, but by 6 wk weight-adjusted intake was higher in the MR group and remained higher (P < 0.05) than the control group thereafter (Fig. 6A). Over the entire study, energy intake of the MR group (178 ± 10 kJ·day−1·100 g BW−1) was 57% higher than controls (113 ± 13 kJ·day−1·100 g BW−1). BW did not differ between groups for the first week, but at all subsequent weeks, BW was significantly lower (P < 0.05) in the MR group compared with controls (Fig. 6B). In fact, the MR group gained only 29 ± 4.7 g during the 6-mo study while the control group gained 296 ± 9.7 g (Fig. 6B). It should be noted, however, that the absolute degree of methionine restriction was not accentuated by the high-fat diet, because the lower methionine per kilojoule intake was more than compensated for by higher energy intake in Osborne-Mendel rats (see Figs. 6A and 3B). The rapid growth and fat deposition of controls consuming ∼60% less energy per unit BW than MR rats (Fig. 6B) illustrates the degree of metabolic inefficiency induced by the MR diet.
Fig. 6.
Postweaning energy intake (A), growth (B), and adiposity (C) of male Osborne-Mendel rats weaned to a high-fat (60 kcal %), methionine-restricted diet. At 32 days of age, rats were randomly assigned to receive a high-fat (60 kcal %) control diet containing 0.86% methionine (control group) or the same high-fat diet with methionine restricted to 0.17% (MR). The composition of the formulated diets is described in the materials and methods, and the diets were provided ad libitum. Food consumption and body weight were measured at weekly intervals, and body composition was determined by DEXA scanning after 8, 16, and 28 wk on the respective diets. The variables were compared by one-way ANOVA at each time point. a,bMeans at each time point with letters that differ denote P < 0.05.
The induction of BAT UCP1 expression by dietary MR approached 4-fold in OM rats, with UCP1 mRNA increasing from 8.8 ± 1.7 fmol mRNA/μg RNA in controls to 31.0 ± 2.5 fmol mRNA/μg RNA in the MR group. This finding is consistent with experiments 1–4 in suggesting that dietary MR increases SNS outflow to BAT. It further suggests that increasing fat content of the diet while restricting methionine does not compromise this component of the response.
DISCUSSION
The present work shows that dietary methionine restriction (MR) produces a reproducible, integrated set of responses in both young and physically mature animals, and in obesity-prone rats consuming a calorically dense formulation of the MR diet. The responses include 1) a substantial long-term increase in total EE that limits fat deposition despite a paradoxical increase in weight-adjusted food consumption, and 2) a pronounced expansion of the dynamic range of RQ between fed and fasted states (e.g., metabolic flexibility). In all experimental settings examined to date, dietary MR increased metabolic flexibility and total EE, with the largest proportion of the increase in EE coming from an exaggerated night-time increase. Thus, the overall effects of MR on metabolism were to enhance insulin sensitivity while simultaneously increasing the energy costs for maintenance of body weight.
Metabolic flexibility measures how effectively substrate switching occurs during the transition from fasting to the fed state. The concept arose from the recognition by Kelley and Mandarino (21) that the shift from fat to carbohydrate utilization was impaired by insulin resistance. The impairment is recognized as a diminution of the normal increase in RQ that occurs upon refeeding and has been attributed to compromised insulin-dependent glucose uptake in peripheral tissues (11–12). The commensurate increase in glucose utilization associated with improved insulin sensitivity is reflected by a concomitant increase of RQ in the fed state. Although hyperinsulinemic-euglycemic clamp data are not yet available, fasting insulin is low and the amount of insulin required to clear a standard glucose load is far less in MR vs. controls (25). Our findings show that the dynamic range of RQ during fasted-to-fed transitions is expanded by dietary MR relative to controls and support the conclusion that metabolic flexibility is significantly enhanced by this dietary regimen.
Growth occurs only when energy intake exceeds the sum of the energy costs for resting EE, heat increment of feeding, locomotion, and facultative thermogenesis (10). The sum is referred to as maintenance energy and was measured historically by determining the equilibrium body weight resulting from a constant fixed intake of a defined diet. Using this approach 40 years ago, Taylor and colleagues (36, 37) developed the concept of efficiency of energy use for maintenance to describe how differences in total EE would affect the body weight maintained at any given rate of food consumption. Thus, at constant intake, animals with higher rates of total daily EE would maintain proportionately lower body weights. Application of Taylor's approach (14) to the weight-stable 20-mo-old rats in experiment 3 yields estimates for maintenance energy costs that were 31.9% higher in the MR group (0.0430 g food·day−1·g BW−1) than controls (0.0326 g food·day−1·g BW−1). EE measured by indirect calorimetry (Table 2) yielded a strikingly similar difference (32.6%) between MR [62.6 ± 0.47 kj·h−1·kg FFM−1] and controls (47.2 ± 0.35 kj·h−1·kg FFM−1). The same principle is evident upon inspection of earlier MR pair-feeding studies (25, 28), where control rats were pair-fed to MR rats using the control diet. At identical energy intakes, MR rats were significantly smaller than controls (25, 28). We conclude that dietary MR increased total daily EE, which translates into higher maintenance costs at all ages from juvenile to adult.
The metabolic responses to dietary MR in young and mature animals share many common features, but three unique differences merit comment. First, when MR is introduced after weaning, the increases in total EE after 3 (∼50%) and 9 mo (∼100%) are significantly larger than the 20–25% increase observed when the MR diet is provided to physically mature animals. However, the increase in total EE after consuming the MR diet for 20 mo after weaning was comparable to the increase when MR was initiated after physical maturity. Second, the effect of MR on day-time EE is age-dependent in the sense that only at 9 mo were both day-time and night-time EE twofold higher in MR vs. control rats, whereas after 3 and 20 mo, the increase in day-time EE accounted for a smaller proportion of the total increase. Third, when instituted after physical maturity, the MR-induced increase in total EE was almost entirely accounted for by the increase in EE at night.
The exaggerated increase in night-time EE and core body temperature in MR rats is consistent with a state of induced thermogenic respiration in brown adipose tissue (15). Fully activated UCP1 can increase basal metabolic rate by as much as 4-fold (16), but a response of this magnitude occurs only during acute cold exposure when the combined transcriptional and posttranscriptional activation of UCP1 is needed to defend body temperature. Previous studies show that transgenic overexpression of UCP1 can also impact energy balance and confer resistance to obesity (22). However, the normal body composition and ability of UCP1 null mice to resist obesity (7, 18) leave open the overall significance of UCP1 in defending body weight. In the present study, dietary MR produced a rapid (within 2 wk) and persistent (9 mo) increase in UCP1 expression in BAT that was correlated with night-time increases in core body temperature at 6 and 12 mo. After 20 mo, MR failed to increase either BAT UCP1 expression or night-time core body temperature. We also found a disconnect between the ability of dietary MR to increase EE and UCP1 expression in β3-adrenergic receptor null mice (29a), and in MR-fed rats treated with bupranolol to block β-adrenergic receptors (Perrone CE, Mallow VL, Krajcik RA, Orentreich N, Gettys TW, unpublished data). In both studies, the absence of an increase in UCP1 mRNA did not compromise the ability of dietary MR to increase total EE and limit fat deposition. These studies indicate that induction of UCP1 expression is not essential to the ability of dietary MR to increase EE, but they also do not rule out the possibility of a contributing role for UCP1 in the variable intensity of the metabolic response to MR observed at different ages.
Another possibility is that UCP1 is a marker for a physiological response to dietary MR that contributes to the overall increase in uncoupled respiration but does not absolutely require the induction of UCP1 to increase EE. For example, UCP1 expression is regulated by sympathetic input through β-adrenergic receptor- and cAMP-dependent mechanisms (1, 17). SNS input also decreases leptin expression (2, 4, 13) and increases oxidative capacity of adipose tissue through targeted effects on gene expression (3, 19). Our data showing that dietary MR increases UCP1 expression in BAT and WAT (Figs. 1, E and F, and Fig. 4, E and F) and affects endocrine function of WAT (Fig. 1D) are fully consistent with the idea that dietary MR increases sympathetic stimulation of adipose tissue. Moreover, our companion paper shows that blocking of β1- and β2-adrenergic receptors in β3-adrenergic null mice with propranolol inhibited over 50% of the night-time increase in EE by dietary MR (29a). Together, our findings suggest that dietary MR impacts EE, in part, through increased SNS signaling to adipose tissue, although the essential response required to increase EE remains unclear.
The goal of understanding how dietary MR uncouples respiration involves identifying its site of action and mechanism. Potential clues are provided in recent studies with cold-adapted UCP1-null mice showing that high rates of uncoupled respiration do not require the presence of UCP1 (40). Activation of thermogenic respiration during cold exposure increases β-oxidation of fatty acids, but it also produces a coordinated increase in glucose utilization coupled with a paradoxical increase in de novo lipogenesis (26, 39, 44). An interesting feature of the interconversion of glucose to lipid prior to oxidation is that it produces more heat and retains less of the potential energy obtained from direct oxidation of glucose (26, 44). Thus, by increasing heat loss and decreasing net ATP generation from glucose during interconversion to lipid, de novo lipogenesis represents a metabolically inefficient substrate cycle capable of making a significant UCP1-independent contribution to nonshivering thermogenesis, particularly during periods of high glucose utilization (26). The interconversion of glucose to lipid prior to oxidation produces a RQ of 1.0 when rates of de novo lipogenesis and lipid oxidation are equal (6, 33). Thus, the impact of this inefficient conversion of glucose to lipid is minimal when rates of fatty acid oxidation are low (e.g., fed state at ambient temperature). However, rates of fatty acid oxidation increased more than 12-fold in UCP1-null mice during cold exposure (40), and night-time RQs reached or exceeded 1.0, guaranteeing that rates of glucose conversion to lipid were proportionately increased to match or exceed the increase in lipid oxidation. Stated another way, the increased flux of glucose through this metabolically inefficient pathway is capable of significantly increasing heat production and EE through a mechanism that does not require but can be enhanced by UCP1. In the present studies, dietary MR produced a consistent enhancement of de novo lipogenesis that was temporally matched with night-time increases in EE. We hypothesize that enhanced substrate cycling of glucose through this mechanism at night is an important component of the uncoupled respiration and metabolic inefficiency produced by dietary MR. It will be important in future studies to measure in vivo rates of de novo lipogenesis and identify the extent of involvement of specific tissues in this potential thermogenic mechanism engaged by dietary MR.
Although our studies provide convincing evidence that dietary MR induces an exaggerated increase in EE, they do not address the role of specific tissues. For example, our studies illustrate that dietary MR alters endocrine function of WAT such that serum leptin and adiponectin are reciprocally affected. While the reduction in serum leptin is consistent with the hyperphagic response to MR, the acute three-fold increase in serum adiponectin observed here and before (25, 29) suggests it as a potential mediator. For example, adiponectin activates AMPK in peripheral tissues (38, 41–43) and stimulates fatty acid oxidation while increasing glucose uptake. Central and peripheral administration of adiponectin also increased EE and decreased body weight (30). The hypothesis that adiponectin may be a mediator of the diet is supported by recent work from our group showing coordinated increases in circulating adiponectin and activation of AMPK in retroperitoneal WAT (29). It will be important in future studies to examine whether adiponectin is required and antecedent to the increase in EE produced by the diet.
We also tested for differences in voluntary activity as a basis for the higher EE in the MR group. Rats typically sleep during the day and consume, on average, only 15–20% of their total daily food intake (8, 23). Voluntary activity increases in the 1- to 2-h period prior to transition from the day-to-night when the remainder of their daily intake of food occurs. We measured voluntary activity at 20 mo in experiment 2 and found no evidence that voluntary activity of the MR group was higher (see Table 3). In fact, ambulatory activity in the MR group was lower than in controls during the day and did not differ from controls at night. Although we cannot rule out group differences in activity at earlier ages, our data from the 20-mo time point provide no evidence that higher activity levels contribute to the higher total EE in the MR group. We have also addressed this issue in studies of dietary MR in younger mice. The findings reported in our companion paper provide no support for voluntary activity as a basis for increases in EE by dietary MR.
A collective reconciliation of the progression of tissue deposition with the components of energy balance provides persuasive evidence that dietary MR induces a chronic state of metabolic inefficiency. The unique effects of the diet on diurnal patterns of EE, body temperature, and fuel selection/oxidation support the view that the metabolic inefficiency involves both uncoupled thermogenic respiration and substrate cycling. Although the present studies were not designed to identify the tissue sites and mechanisms engaged by dietary MR, they do provide a clear framework and compelling rationale for addressing these questions in future studies. For example, given the compartmentalization and highly integrated function of liver, adipose tissue, and muscle metabolism during the daily transitions between fed and fasted states, the metabolic remodeling produced by dietary MR is likely to involve changes in the way these tissues function together. An important future goal will be to identify the tissues where substrate cycles are engaged by dietary MR and assess their relative impact on overall in vivo energy expenditure.
Perspectives and Significance
The present studies provide compelling evidence that dietary MR increases metabolic flexibility and limits fat accretion by chronically increasing the energy costs of body weight maintenance. These responses are components of a beneficial metabolic phenotype that provides both short- and long-term benefits, as evidenced by previous work showing that dietary MR extends lifespan by an average of 30% (27–28, 31). The broader implication of these findings is that the increased rate of respiration produced by dietary MR does not accelerate aging in the manner predicted by the metabolic rate and rate of living hypotheses (32, 34). The present work implies that the health and longevity of rodents, and perhaps other species, could be improved by lowering dietary methionine. This would be difficult to achieve in practice because of the methionine and cysteine content of grains used to formulate rodent diets. However, a better understanding of the nutrient-sensing and signaling mechanisms that link reduction in dietary methionine to regulation of energy homeostasis could provide insights into novel approaches to beneficially modulate these communication networks in other ways.
GRANTS
This work was supported by the Orentreich Family Foundation, and, in part, by the National Center for Complementary and Alternative Medicine (P50-AT 002776-01) and by National Institutes of Health (NIH) grant P20-RR 021945 (to T. W. Gettys) from the National Center for Research Resources, NIH Nutrition Obesity Research Center Grant 1P30 DK 072476, and NIH RO1 074772 (T. W. Gettys).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Aaron Adamson, Amanda Laque, and Jeri Gomez (Pennington Biomedical Research Center) for excellent technical contributions and Dr. Michael Pellizzon for advice in formulating the diets (Research Diets). We thank Anne Gooch and Meghan Greeley for administrative support.
REFERENCES
- 1.Bouillaud F, Ricquier D, Mory G, Thibault J. Increased level of mRNA for the uncoupling protein in brown adipose tissue of rats during thermogenesis induced by cold exposure or norepinephrine infusion. J Biol Chem 259: 11583–11586, 1984 [PubMed] [Google Scholar]
- 2.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter RD, Gettys TW. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology 140: 4772–4776, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Commins SP, Watson PM, Frampton IC, Gettys TW. Leptin selectively reduces white adipose tissue in mice via an uncoupling protein-1-dependent mechanism. Am J Physiol Endocrinol Metab 280: E372–E377, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different β-adrenoceptor subtypes. J Biol Chem 275: 33059–33067, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 140: 292–300, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Elia M, Livesey G. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr 47: 591–607, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism 37: 287–301, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Flatt JP. Dietary fat, carbohydrate balance, and weight maintenance. Ann NY Acad Sci 683: 122–140, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Galgani JE, Heilbronn LK, Azuma K, Kelley DE, Albu JB, Pi-Sunyer X, Smith SR, Ravussin E. Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 57: 841–845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295: E1009–E1017, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettys TW, Harkness PJ, Watson PM. The β3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology 137: 4054–4057, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Gettys TW, Mills S, Henricks DM. An evaluation of the relationship between food consumption rate and equilibrium body-weight in male rats. Br J Nutr 60: 151–160, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr 28: 13–33, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Himms-Hagen J. Regulation of metabolic processes in brown adipose tissue in relation to nonshivering thermogenesis. Adv Enzyme Regul 8: 131–151, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Himms-Hagen J. Brown adipose tissue metabolism and thermogenesis. Ann Rev Nutr 5: 69–94, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem 276: 12460–12465, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hwa JJ, Fawzi AB, Graziano MP, Ghibaudi L, Williams P, Van Heek M, Davis H, Rudinski M, Sybertz E, Strader CD. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol Regul Integr Comp Physiol 272: R1204–R1209, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Jequier E, Felber JP. Indirect calorimetry. Baillieres Clin Endocrinol Metab 1: 911–935, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 96: 2914–2923, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Shen L, Liu Y, Tajima D, Sakai R, Woods SC, Tso P. Diurnal rhythm of apolipoprotein A-IV in rat hypothalamus and its relation to food intake and corticosterone. Endocrinology 145: 3232–3238, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr 47: 608–628, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5: 305–314, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Masoro EJ. Role of lipogenesis in nonshivering thermogenesis. Fed Proc 22: 868–873, 1963 [PubMed] [Google Scholar]
- 27.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4: 119–125, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 123: 269–274, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Perrone CE, Mattocks DA, Hristopoulos G, Plummer JD, Krajcik RA, Orentreich N. Methionine restriction effects on 11-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J Lipid Res 49: 12–23, 2008 [DOI] [PubMed] [Google Scholar]
- 29a.Plaisance EP, Henagan TM, Echlin H, Boudreau A, Hill KL, Lenard NR, Hasek BE, Orentreich N, Gettys TW. Role of β-adrenergic receptors in the hyperphagic and hypermetabolic responses of dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol (June16, 2010). doi:10.1152/ajpregu.00838.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med 10: 524–529, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8: 1302–1307, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Sacher GA, Duffy PH. Genetic relation of life span to metabolic rate for inbred mouse strains and their hybrids. Fed Proc 38: 184–188, 1979 [PubMed] [Google Scholar]
- 33.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol Endocrinol Metab 258: E399–E412, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci 35: 75–104, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A, Majewski M, Cefalu WT, Gettys TW. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metab Clin Exp 57: S39–S46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor SCS, Turner HG, Young GB. Genetic control of equilibrium maintenance efficiency in cattle. Anim Prod 33: 179–194, 1981 [Google Scholar]
- 37.Taylor SCS, Young GB. Equilibrium weight in relation to food intake and genotype in twin cattle. Anim Prod 10: 393–412, 1968 [Google Scholar]
- 38.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99: 16309–16313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trayhurn P. Fuel selection in brown adipose tissue. Proc Nutr Soc 54: 39–47, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem 281: 31894–31908, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13: 332–339, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55: 2562–2570, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Yu XX, Lewin DA, Forrest W, Adams SH. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J 16: 155–168, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, Mynatt RL, Gettys TW. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J 19: 1482–1491, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol 38: 47–52, 2003 [DOI] [PubMed] [Google Scholar]