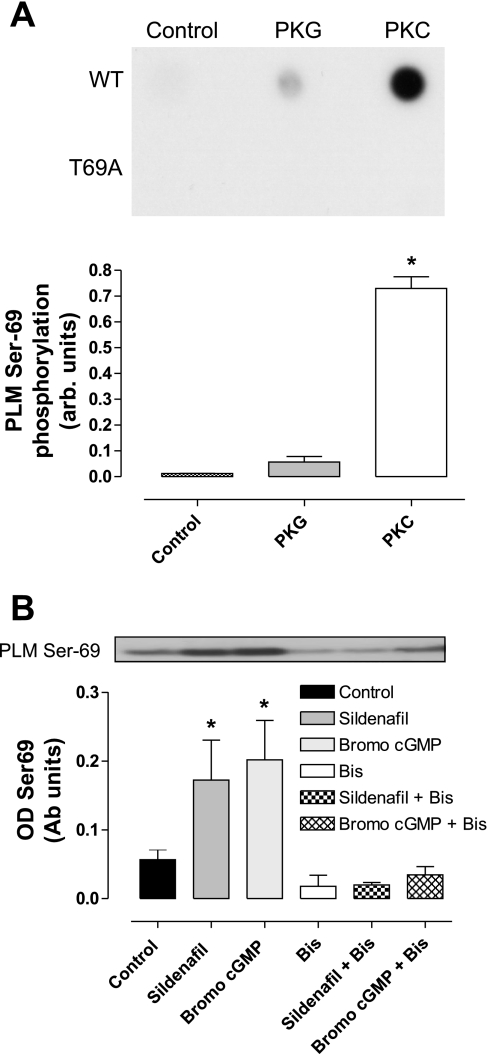
Fig. 6.
A: the ability of PKG or PKC to phosphorylate a recombinant PLM peptide in vitro was assessed using WT or a T69A (which cannot be phosphorylated at that site) recombinant peptide. Dot blots were probed with an anti-PLM CP69 antibody to index phosphorylation. The T69A serves as a control that shows the phosphospecific antibody only recognizes modification of Ser/Thr69, and does not cross-react with other sites. Whilst PKC efficiently phosphorylated the PLM peptide, PKG did not. From previous HPLC studies (not shown), we know that the PKC-dependent signal represents a stoichiometric phosphorylation of the PLM peptide. Arb units, arbitrary units. B: the effect of PKC inhibition on PLM Thr69 phosphorylation in cultured adult rat ventricular myocytes. Sildenafil or 8-bromo-cGMP each increased PLM Thr69 phosphorylation, and this was robustly blocked by the PKC inhibitor bisindolylmaleimide (Bis). OD, optical density; Ab units, arbitrary units. Representative blots are shown with quantitative analysis of repeat experiments. Bars represent means ± SE; n = 3–5 experiments. *P < 0.05 vs. control (1-way ANOVA).