FIG. 1.
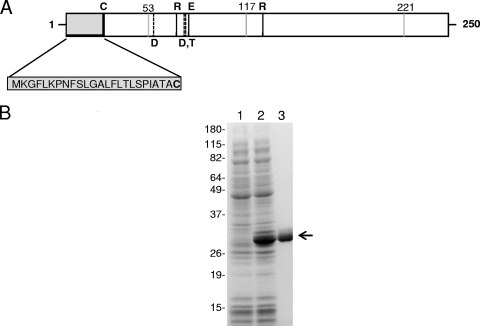
MG_186 organization, cloning, expression, and purification. (A) Schematic of MG_186. MG_186 is comprised of 250 amino acids and contains a hydrophobic amino-terminal signal sequence and prokaryotic lipoprotein cleavage site (indicated by the gray box and the letter C, respectively). Amino acids 44 through 200 have significant identity and similarity with the thermonuclease domain profile first described for the Nuc thermonuclease of Staphylococcus aureus (11, 23, 36). Conserved amino acid residues involved in binding of calcium ions (dashed vertical lines) are aspartate (D57, D77) and tyrosine (T78). Amino acid residues arginine (R72, R126) and glutamate (E80) are also conserved and comprise the active catalytic site (black vertical lines). Positions of UGA-encoded tryptophans (53, 117, and 221) are indicated by gray vertical lines. (B) Expression and purification of rMG_186. The MG_186 gene without its signal peptide was cloned, expressed, and purified as a His-tagged protein as detailed in Materials and Methods. Protein fractions were resolved on 4 to 12% NuPAGE gradient gels. Lane 1, uninduced E. coli pET19b-MG186 total cell lysate; lane 2, induced E. coli pET19b-MG186 total cell lysate; and lane 3, Ni-NTA column-purified rMG_186. The arrow indicates rMG_186. Molecular weight markers are indicated on the left.