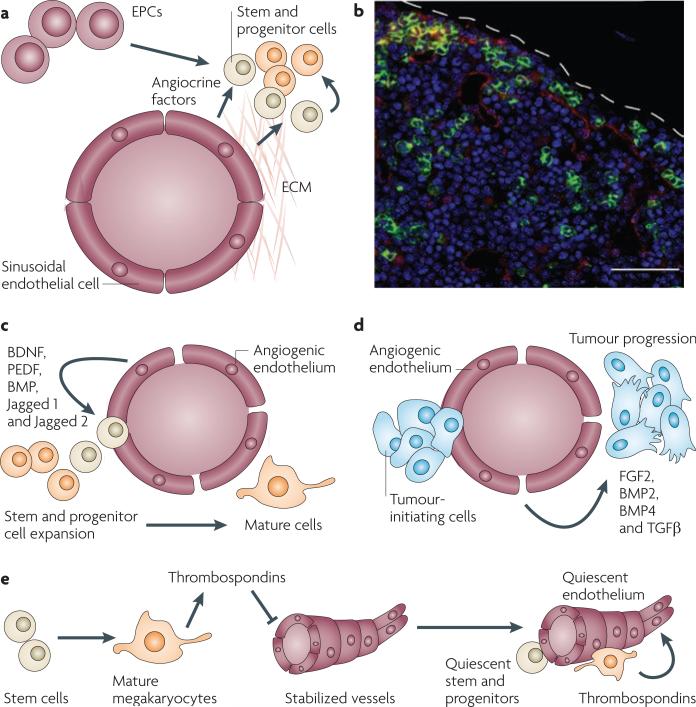
Figure 1. The vascular niche supports the expansion of stem and progenitor cells as well as their malignant counterparts.
a | By expressing angiocrine factors and producing extracellular matrix (ECM), endothelial cells and endothelial progenitor cells (EPCs) establish a microenvironment, referred to as the vascular niche, which supports the expansion of normal and malignant stem and progenitor cells. b | Establishment of the vascular niche by the bone marrow sinusoidal endothelial cells. The confocal image shows a cross-section of the bone marrow taken from transgenic Notch reporter mice 7 days after sublethal irradiation with 6.5 Gy. Regenerating Notch-activated green fluorescent protein (GFP)+ haematopoietic stem and progenitor cells (green fluorescence) could be detected in the proximity of VE-cadherin+ Notch ligand+ sinusoidal endothelial cells (red staining). Therefore, angiocrine factors, such as Notch ligands, reconstitute haematopoiesis. c | Vascular endothelial growth factor A (VEGFA)- or fibroblast growth factor 2 (FGF2)-activated endothelial cells promote the proliferation and lineage-specific differentiation of normal cells by release of angiocrine factors (FGFs, BMPs, jagged 1 and jagged 2) and direct cellular contact. d | Identical pathways to those described in (c) affect malignant stem and progenitor cells. e | The mature progeny of stem cells, such as megakaryocytes, enforce a quiescent state in the endothelial cells by inhibiting their angiogenic activity through the release of anti-angiogenic factors, such as thrombospondins, which in turn promotes stem cell quiescence. BDNF, bone-derived neurotrophic factor; BMP, bone morphogenetic protein; PEDF, pigment epithelium-derived factor; TGFβ, transforming growth factor-β. Bar represents 50 μm.