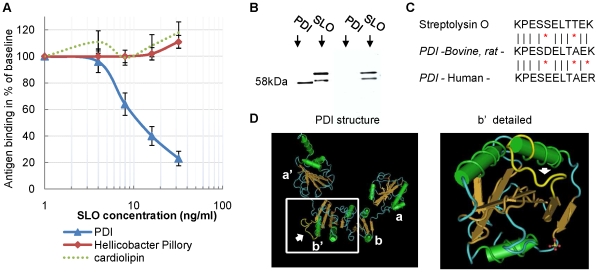
Figure 2. Mimicry between PDI and Streptolysin O (SLO).
(A) Competitive ELISA demonstrates a common SLO-PDI determinant. Serum samples (n = 20) positive for anti-PDI, anti-Helicobacter Pillory (HP) and anti-cardiolipin (ACL) antibodies were pre-incubated with increasing amounts of SLO (0–32 ng/µl) and subjected to ELISAs (PDI, HP and ACL). Baselines (100%) were determined by incubation of each serum with PBS only. Results are presented as a mean ± SEM. (B) Reactivity of ASLO antibodies with and without anti-PDI activity. Purified bovine PDI (100 ng) and purified SLO (10 ng) were subjected to SDS gel electrophoresis followed by immunoblotting with Affinity purified anti-SLO antibodies from anti-PDI positive (left) – and anti-PDI negative (right) subjects. (C) Similar immunogenic determinants in SLO and PDI. The 11 amino-acids sequence of the similar determinant is presented in SLO (p51–p61), bovine/rat PDI – (p330-340) and human PDI (p328-338). (D) Location of the similar determinant on the PDI protein (in yellow, pointed by an arrow). Left – structure of all 4 subunits (yeast). Right – focus on the b' subunit (human), an important subunit containing the substrate binding site. Note that the similar determinant is in the exposed area of the binding site. In the SLO protein, the determinant is also exposed (p51-61; not shown).