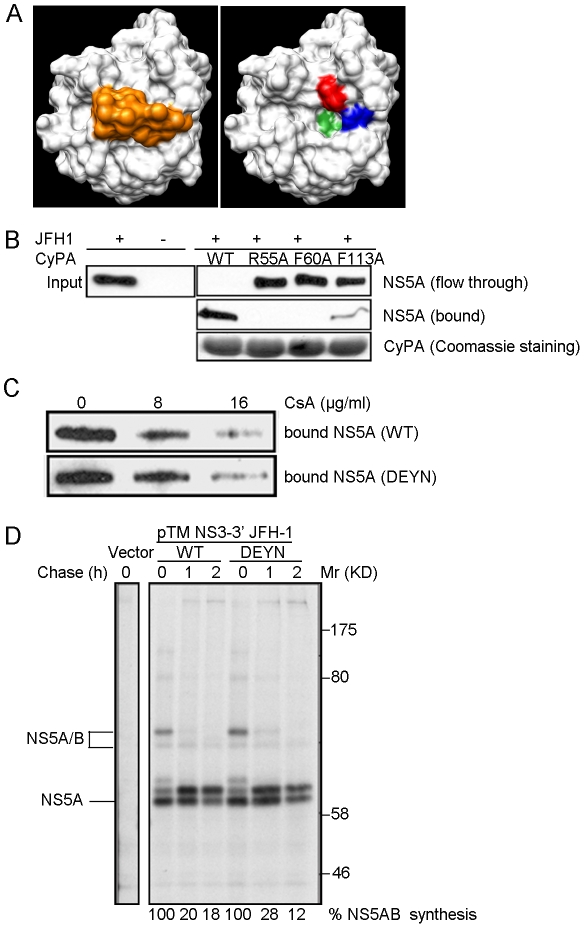
Figure 4. DEYN mutations do not affect NS5A binding or NS5A-5B cleavage.
(A) Structure of CyPA. Left panel: Space-fill model of CyPA-CsA complex (2RMA) [52]. White: CyPA; orange: CsA. Right panel: residues in the active site of CyPA that are mutated in this study. Red: R55; blue: F60; green: F113. (B) In vitro binding of CyPA to full-length NS5A. His-tagged CyPA proteins was incubated with infected cell lysate and then subjected to Ni-NTA column pull-down. CyPA-NS5A complexes were eluted with Imidazole (bound) and then subjected to immunobloting with anti-NS5A antibodies. Result shown is representative of two independent experiments. (C) WT and DEYN mutant NS5A protein exhibit similar CsA sensitivity in NS5A binding. Binding reactions were performed as described in (B) with increasing amount of CsA present. (D) Cleavage kinetics at NS5A-5B site, depicted by pulse-chase labeling and immunoprecipitation. NS5AB protein bands were quantified by densitometry, and we calculated viral protein synthesis by dividing the values obtained for the chase samples by the corresponding values obtained from the 0 h chase samples. Molecular weights are shown at the right; lines point to the respective HCV proteins.