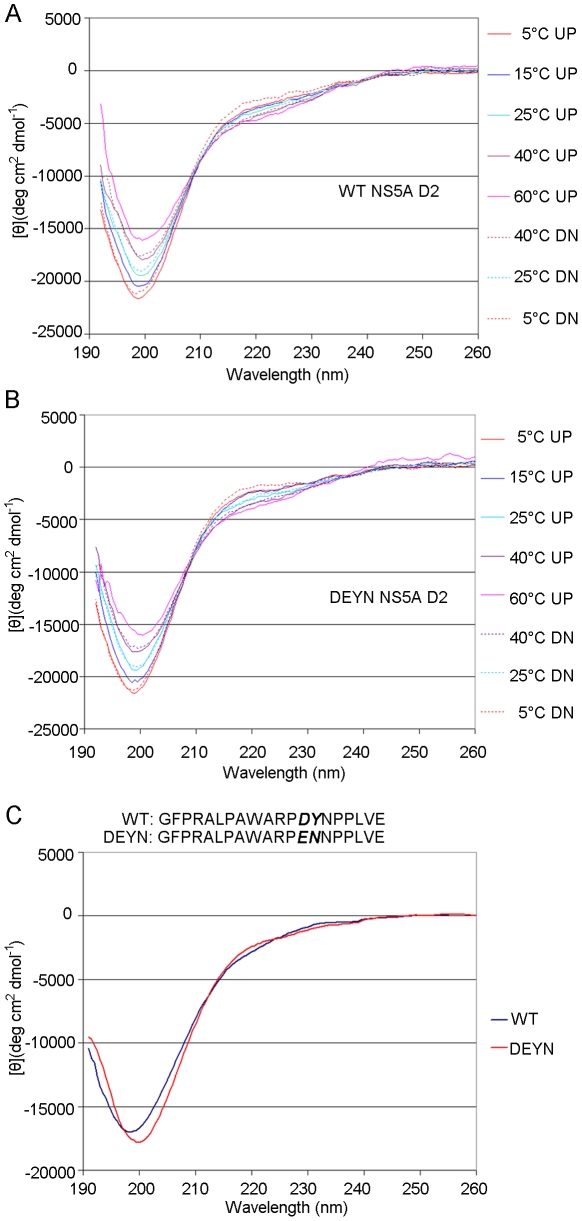
Figure 5. Circular Dichroism analysis of WT and DEYN NS5A D2 and peptides.
(A) Thermal unfolding of wt NS5A D2 reveals an isodichroic point at approximately 208 nm indicative of a β-turn/extended structure conformational transition [53], [54]. Solid lines represent spectra collected with increasing temperature (UP), with dashed lines showing reversibility of the unfolding process upon subsequent cooling of the wt NS5A D2 sample (DN). (B) Similar results were obtained for DEYN NS5A D2. (C) The circular dichroism spectra of the WT and DEYN mutant peptides exhibit features characteristic of largely unfolded conformations. The difference in spectral magnitude and a slight shift in the position of the spectral minimum, however, indicate a conformational difference between the WT and DEYN peptides. Result shown is representative of five independent experiments.