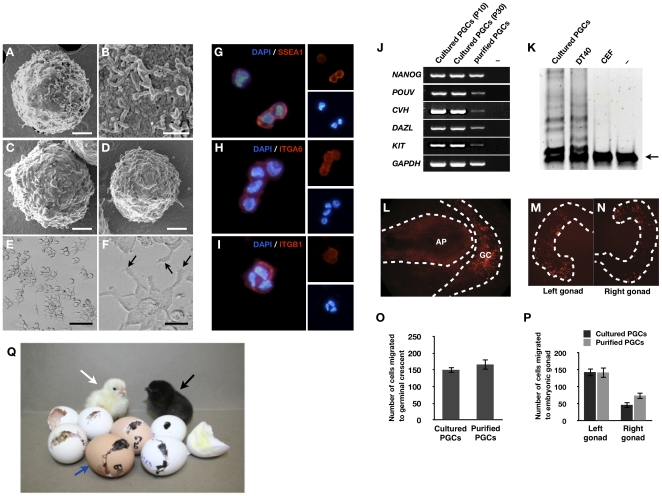
Figure 3. Characterization of cultured PGCs.
Scanning electron microscopy of cultured PGCs (A–B), blood PGCs (C), and gonadal PGCs (D). (E, F) PGCs cultured on Matrigel (arrows indicate pseudopod-like structures). Bars: 2 µm (A, C, and D); 200 nm (B); 100 µm (E); and 25 µm (F). (G–I) Immunocytochemical analysis of cultured PGCs. PGCs cultured for 60 days were immunostained with antibodies raised against SSEA-1 (G), ITGA6 (H), and ITGB1 (I). (J) RT-PCR analysis of NANOG, POUV, CVH, DAZL, and KIT in cultured PGCs (passages 10 and 20) [–: negative control (no template)]. (K) Telomerase activity in PGCs. Repeated sequences were observed in PGCs and DT40 (positive control cells). Chicken embryonic fibroblast (CEF) and buffer (–) were used as negative controls. Arrow indicates the 56-bp internal control template band. (L) Migration of cultured PGCs into the germinal crescent. Approximately 500 PGCs, cultured for 82 days or purified by MACS, were labeled with PKH26 and then transferred into the subgerminal cavities of blastoderm embryos. Labeled cells (red) were detected in the germinal crescent (arrows) (GC: germinal crescent, AP: area pellucida). (M–N) Gonadal migration of culture PGCs. Approximately 500 PGCs, cultured for 82 days or purified by MACS, were labeled with PKH26 and then injected into blood vessels of recipient embryos at stage 14–17. Labeled cells (red) were detected in the embryonic gonad. (O) Numbers of PGCs that had migrated into the germinal crescent of stage 6 embryos that had, as stage X recipient embryos, been injected with 500 PGCs (mean ± SEM; n = 12 for purified PGCs, n = 11 for cultured PGCs). No significant difference was observed between cultured and purified PGCs. (P) Number of PGCs that had migrated into the gonads of stage 28 embryos that had, as stage 14 recipient embryos, been injected (i.v.) with 500 PGCs (mean ± SEM; n = 12 for purified PGCs, n = 10 for cultured PGCs). No significant difference was observed between cultured and purified PGCs. Statistical analysis was conducted with the general linear model (PROC-GLM) of SAS software. (Q) Germline transmission of cultured PGCs. Donor KO (i/i) PGCs cultured for more than 50 days were injected into the dorsal aorta of WL (I/I) recipient embryos (female), and after sexual maturation, progeny were derived from the donor KO PGCs (black plumage color, black arrow). Progeny that derived from the endogenous WL PGCs are noted by a white arrow. The white egg indicates that the recipient chicken is WL because KO chickens lay brown eggs as indicated by the blue arrow.