Table 2.
Alcohol Addition Scope
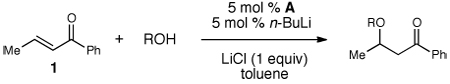 | |||
|---|---|---|---|
| entry | R | product | yield(%)b |
| 1 | Bn | 2 | 89 |
| 2 | Me | 3 | 79 |
| 3 | allyl | 4 | 78 |
| 4 | propargyl | 5 | 85 |
| 5 | 4-MeO-C6H4CH2OH | 6 | 81 |
| 6 | 7 | 80 | |
| 7 | 8 | 75 | |
| 8c | 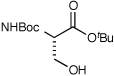 |
9 | 81d |
| 9 | 10 | 75d | |
| 10 | 11 | 89 | |
See Supporting Information for details.
Isolated yields.
CH2Cl2 used as solvent.
1.2:1 dr (determined by 1H NMR spectroscopy).
